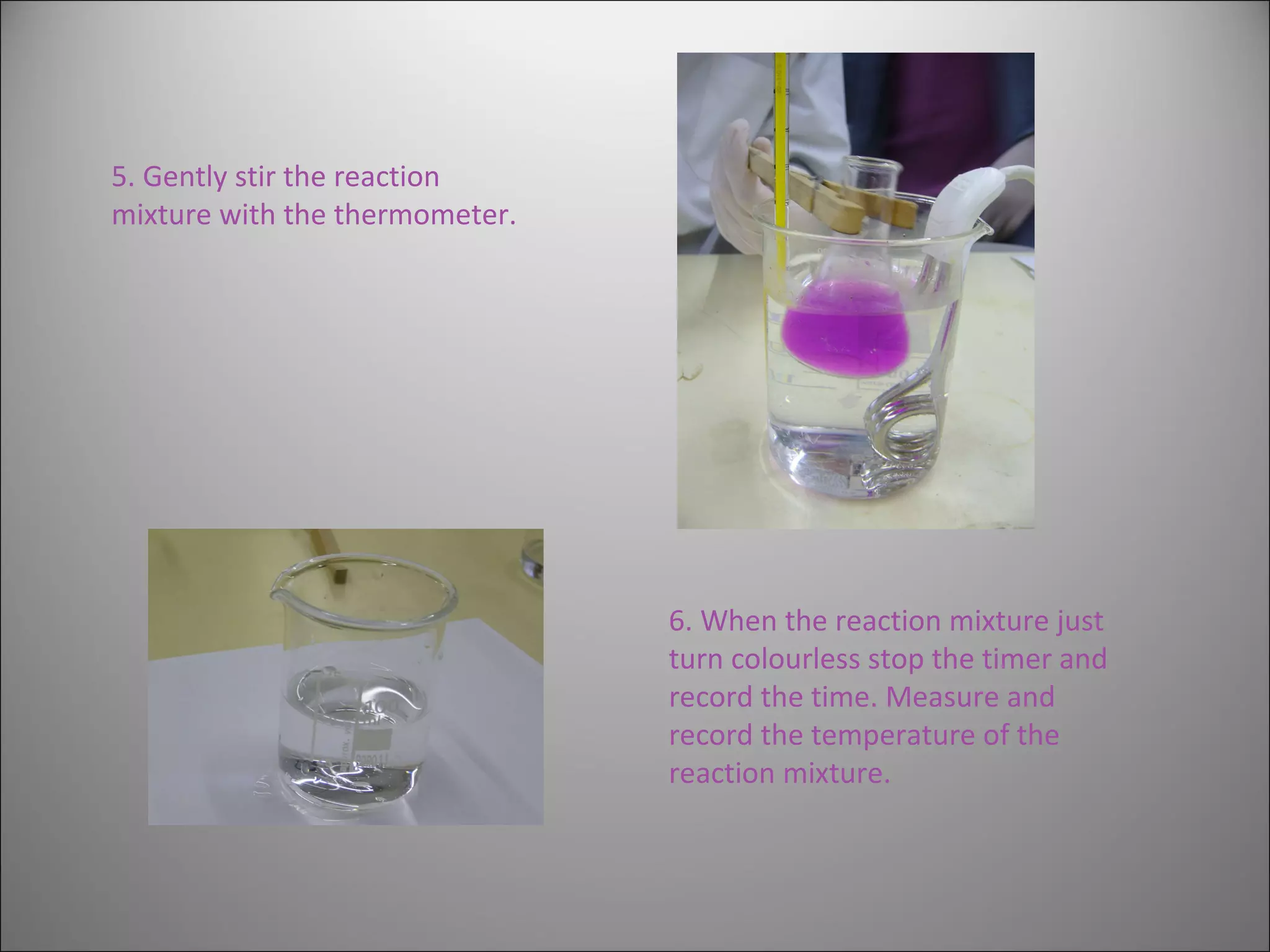
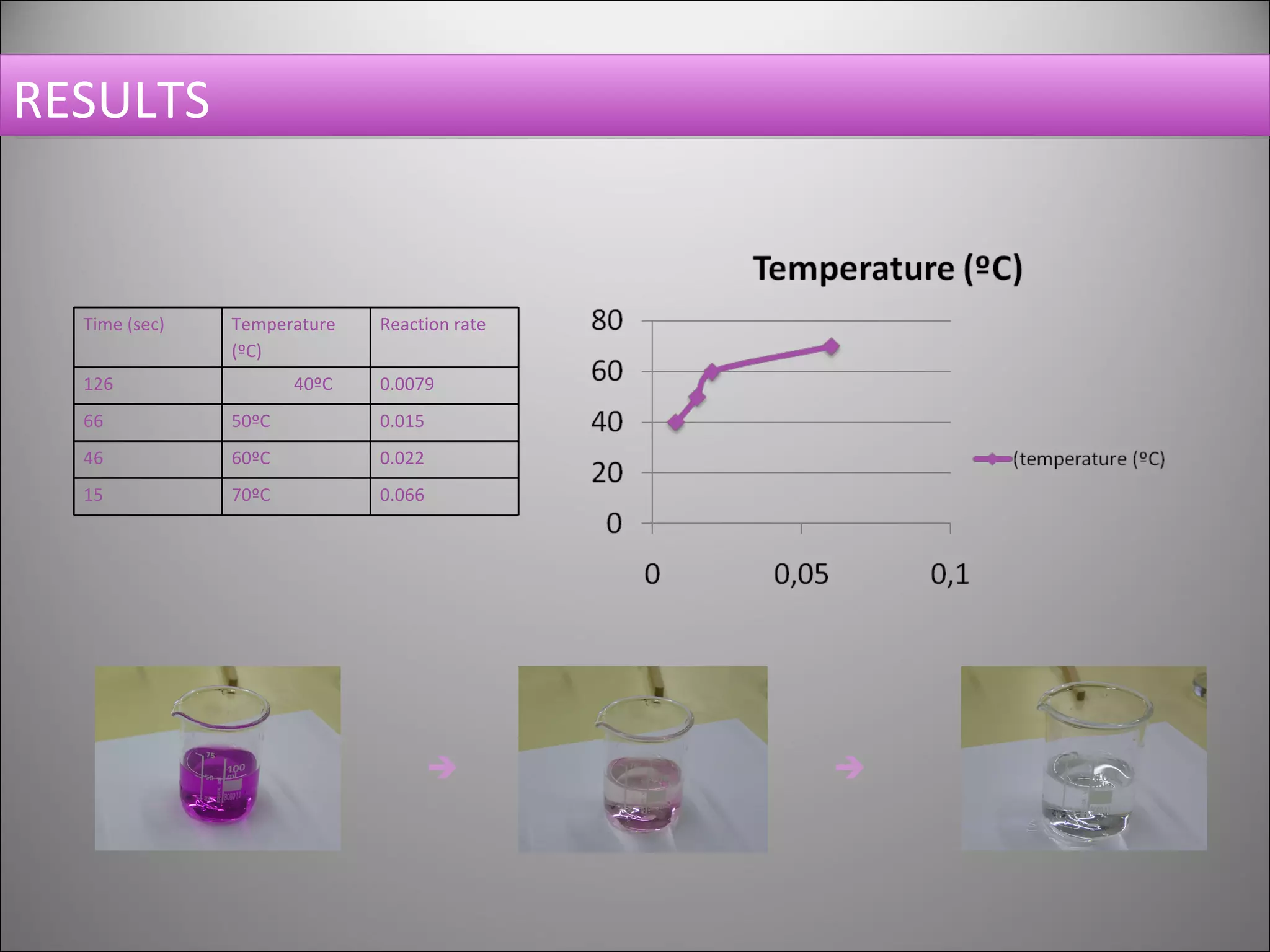
This document summarizes an experiment that investigates the effect of temperature on reaction rate. The experiment uses the reaction between potassium permanganate and oxalic acid, where the disappearance of the purple permanganate color indicates the extent of reaction. The time it takes for the color to disappear at different temperatures (40, 50, 60, 70°C) is measured and the reaction rate is calculated as the inverse of time. The results show that increasing the temperature leads to faster reaction rates, demonstrating that temperature has an activating effect on reaction kinetics.