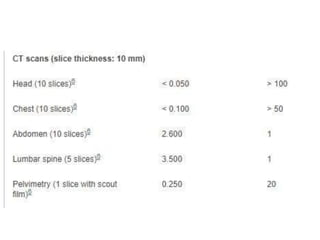
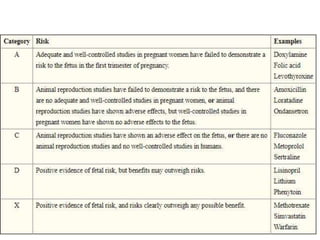
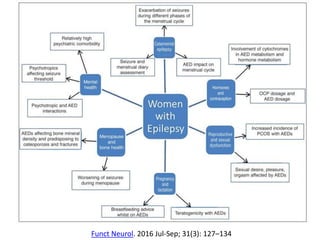
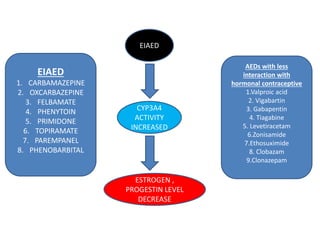
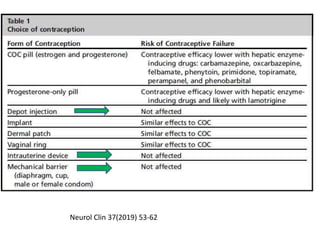
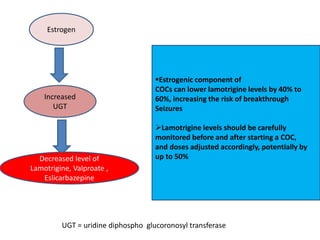
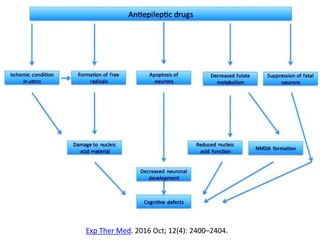
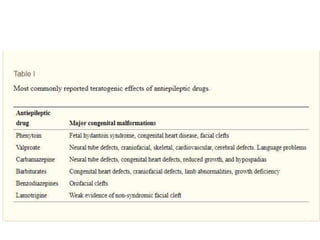
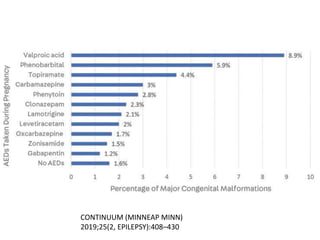
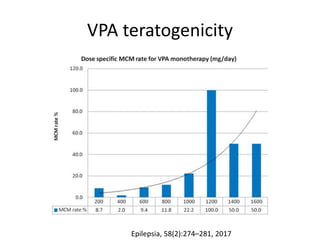
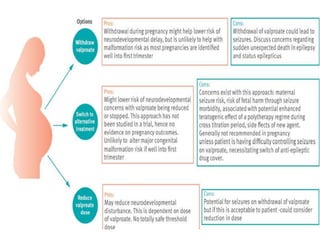
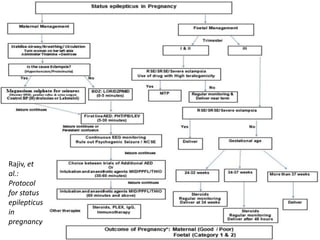
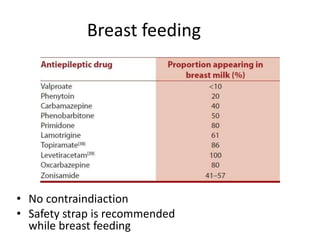
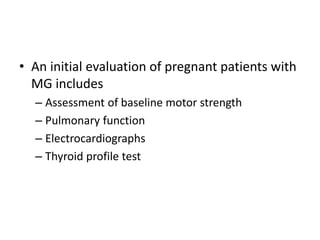
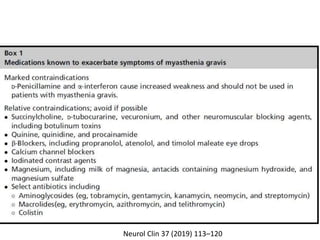
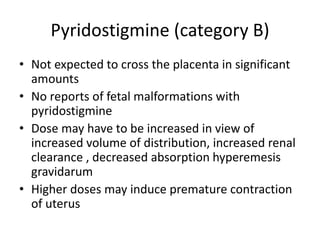
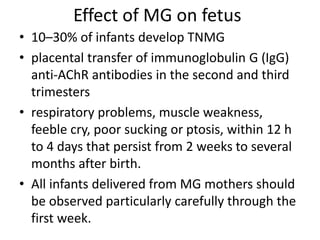
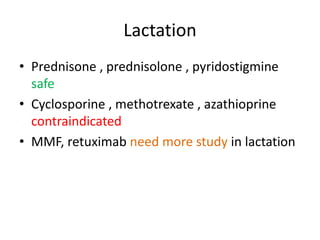
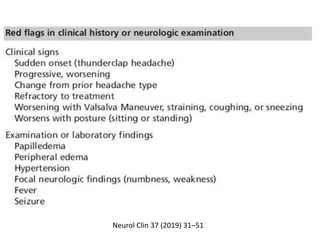
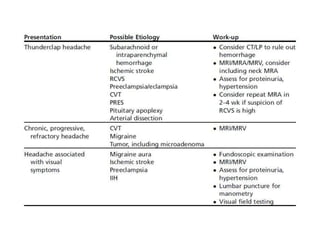
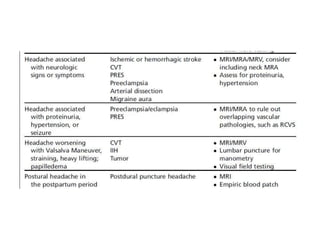
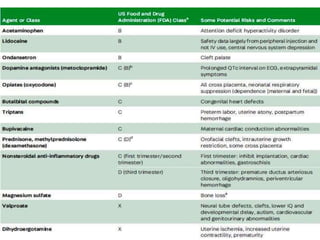
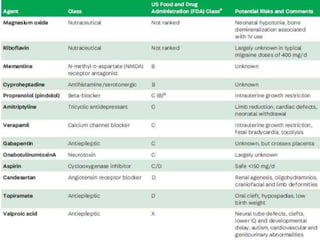
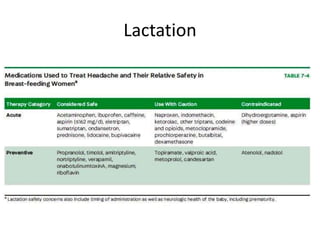
This document provides an overview of neurology topics related to pregnancy, including diagnostic imaging, pre-existing neurological diseases like epilepsy and myasthenia gravis, and their management during pregnancy. It discusses safety of different imaging modalities in pregnancy, effects of pregnancy on diseases and their treatment, risks to the mother and fetus, and recommendations to minimize risks. Medication management is addressed for various conditions, focusing on maintaining seizure control and myasthenia gravis remission while minimizing fetal risks.