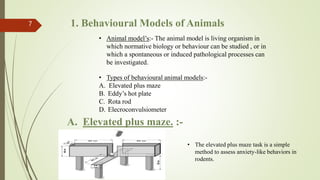
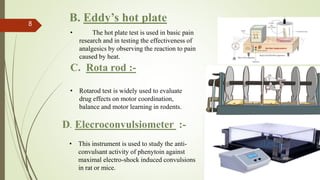
The document discusses the importance of preclinical pharmacological studies in drug development, which involves research conducted before human clinical trials to assess drug safety, efficacy, and potential toxicity, primarily using laboratory animals. It outlines various animal models, methodologies, and assays used to evaluate drug candidates, including behavioral models and biochemical tests, to provide necessary data for FDA submissions. The role of preclinical studies in determining dosage, identifying lead candidates, and ensuring the safety of new drugs is emphasized.









![4.Protein Estimation :-
12
Proteins estimations or protein assays are done to estimate the
concentration or amount of protein present in product.
A. Biuret method:-
Principle :-
Protein reacts with this alkaline copper complex and colour changes to violet.
The protein can then be estimated by reading the absorption at 540nm. This
method takes 20-30 minutes to complete.
Principle :-
The principle behind the Lowry method of determining protein Concentrations lies in
the reactivity of the peptide nitrogen[s] with the copper[II] ions under alkaline
conditions.
B. Lowry Method :-](https://image.slidesharecdn.com/akashkalepracticeschool-220521165711-c5b057ef/85/Preclinical-Pharmacological-Experiments-pptx-12-320.jpg)




