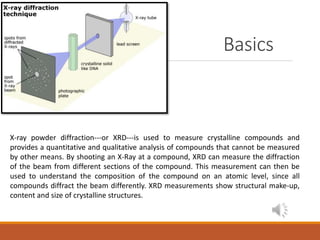
X-ray powder diffraction (XRD) is used to analyze crystalline compounds on an atomic level. By shooting an X-ray beam at a sample, XRD can measure how the beam is diffracted by different sections of the compound, providing information about its composition, structure, and crystalline size. The basic components of an XRD instrument include an X-ray source like an X-ray tube or radioactive isotope, a wavelength selector using filters or monochromators, a sample holder that rotates the sample, and an X-ray detector like a gas detector, scintillation counter, or semiconductor detector.