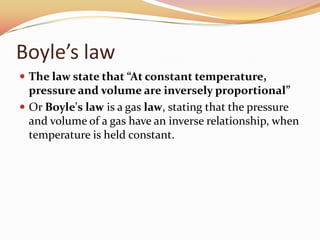
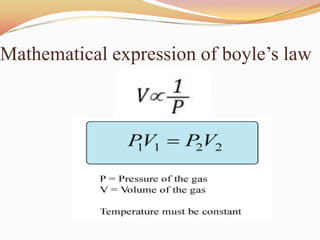
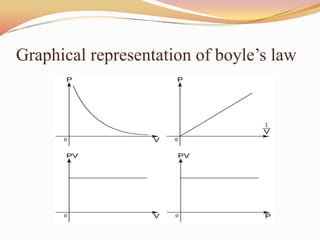
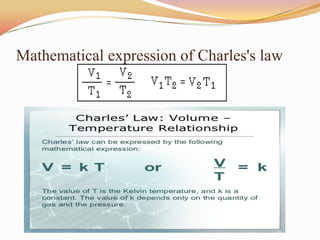
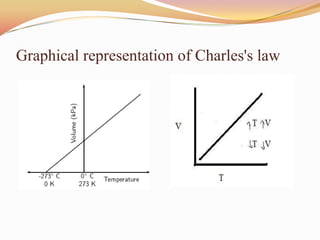
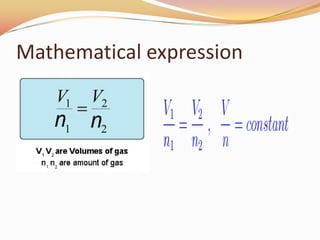
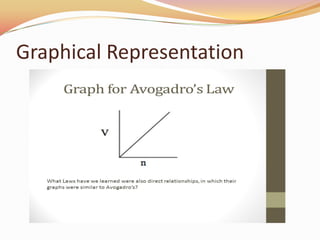
This document discusses three gas laws: Boyle's law, Charles's law, and Avogadro's law. Boyle's law states that at a constant temperature, the pressure and volume of a gas are inversely proportional. Charles's law describes how gases expand when heated, with temperature and volume being directly related at constant pressure. Avogadro's law establishes that equal volumes of gases at the same temperature and pressure contain equal numbers of molecules.