This study aimed to validate the diagnostic criteria for Parkinson's disease (PD) established by the International Parkinson and Movement Disorder Society (MDS) against expert clinical diagnosis. It involved 626 participants, with the MDS criteria showing a sensitivity of 94.5% and specificity of 88.5%, outperforming the UK Brain Bank criteria in both measures. The results indicate that the MDS criteria provide a reliable framework for diagnosing PD in clinical and research settings.


![the assessments without required reference to the
expert neurologist’s diagnosis and were not asked to
provide their diagnostic opinion; rather, they were
simply instructed to evaluate whether each individ-
ual criterion (from a checklist) was met. The evalua-
tor used both interview and in-person examination
to document criteria. In addition, chart review could
be performed to document features not available on
current history and examination. Based on the
review, each individual criterion was scored by the
rater as present or absent. The approximate time
taken to complete the checklist was 15 minutes for
each scale.
As olfactory testing is included as a supportive crite-
rion in the MDS-PD criteria, it was systematically tested
in this study (10 patients also had metaiodo-
benzylguanidine [MIBG] scintigraphy results available).
Centers used either the 12-item Brief Smell-
Identification test version A (BSIT) 6,7
or the Sniffin
Sticks.8
Age-standardized normal values were taken
from the published literature.6,8
Abnormal olfaction
was defined as a score below the 10th percentile for
age. The Sniffin Sticks combine all patients > 55 years
together in 1 category; therefore, for consistency, the
same standardization was performed for the BSIT (ie,
cutoff of 6). On secondary analysis, a second adjust-
ment subdivided the >55 age group on BSIT. Blood
pressure was measured in the supine position and then
reassessed standing after 3 minutes.
Data Analysis
The primary outcome was the accuracy/concordance
of MDS criteria according to the gold standard, clinical
diagnosis. Sensitivity and specificity, as well as kappa
agreement, were calculated for each measure. Compari-
sons between MDS and UK Brain Bank criteria were
tested with the Fisher exact test. The prevalence of each
individual criterion was assessed in the PD and non-PD
groups. Prespecified secondary analyses included analy-
sis of those with high (>80%) versus low (≤80%) cer-
tainty of PD and those with short (<5 years) versus
longer (≥5 years) disease duration. Additional second-
ary analyses assessed the effect of age and sex, and ana-
lyses testing different permutations of criteria were
performed (see Results section). Statistical tests were
performed with SPSS software, version 22.
Results
Patient Characteristics
Overall, we recruited 626 patients: 434 with PD and
192 with non-PD parkinsonism (Table 1). Age and sex
were similar between groups. As expected, disease
duration was shorter in the non-PD group (2.8 vs
6.3 years). In the non-PD group, the commonest condi-
tions were multiple system atrophy (38%), progressive
supranuclear palsy (33%), and corticobasal syndrome
(10%); see Supplemental Table 1.
Overall Performance of MDS Criteria
Of the 434 patients diagnosed with PD by the gold-
standard expert, 94.5% met MDS criteria for probable
PD (ie, 5.5% false-negative rate). Of the non-PD
patients, 88.5% were identified as non-PD by the cri-
teria (ie, 11.5% false-positive rate). Combining all
patients, the overall accuracy for probable PD was
92.6% compared with the gold standard, expert clinical
diagnosis. In addition, 59.3% of PD patients met MDS
criteria for clinically established PD. Only 1.6% of
non-PD patients met clinically established criteria.
In comparison, the UK Brain Bank had both lower
sensitivity and specificity. 89.4% of PD patients met
UK Brain Bank criteria for PD (10.6% false-negatives,
P = 0.008 to MDS criteria), and 79.2% of non-PD
patients were identified as non-PD by UK Brain Bank
criteria (20.8% false-positives, P = 0.018). Overall
accuracy was 86.4%, significantly lower than the MDS
rate (P < 0.001). Removing the dementia exclusion
from the UK Brain Bank criteria (to account for defini-
tion changes3,5
) made no difference to the results (false-
negatives, 11.4%; false-positives, 21.4%). A high pro-
portion of PD patients (83.9%) met UK Brain Bank cri-
teria for definite PD, with a false-positive rate of 8.9%
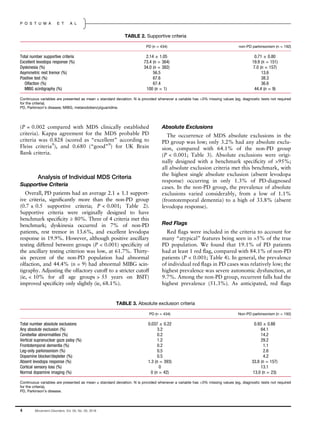
TABLE 1. Patient characteristics and overall criteria performance
PD (n = 434) non-PD parkinsonism (n = 192)
Age 66.6 ± 9.7 68.0 ± 9.9
Sex (% female) 64 60
Disease duration from symptom onset (years) 7.5 ± 6.0 4.3 ± 2.9
Disease duration from diagnosis (years) 6.3 ± 5.8 2.8 ± 2.4
Meets MDS probable criteria (%) 94.5 11.5
Meets UK Brain Bank probable (%) 89.4 20.8
Meets MDS clinically established (%) 60.4 1.6
Meets UK Brain Bank definite (%) 83.9 8.9
Continuous variables are presented as mean ± standard deviation.
PD, Parkinson’s disease; MDS, International Parkinson and Movement Disorder Society; UK, United Kingdom.
Movement Disorders, Vol. 00, No. 00, 2018 3
V A L I D A T I O N O F M D S C R I T E R I A](https://image.slidesharecdn.com/postuma2018-190801223829/85/Postuma2018-3-320.jpg)

![6. Effect of age: Age is a potential confounder in diagno-
sis, as comorbid conditions and multiple neurodegen-
erative pathologies are more common. We divided
each group according to age above or below the
group median. Sensitivity did not differ (93.4% young
vs 95.4% old), but specificity was lower in older
patients (93.8% vs 83.3%, P = 0.039).
Testing Potential Modifications
1. Although the ancillary testing supportive criterion
did not meet the 80% specificity threshold in our
study, removing this from the diagnostic criteria did
not improve overall accuracy (91.3% without ancil-
lary testing vs 92.7% with testing). Sensitivity
dropped from 94.5% to 91.9%, whereas specificity
rose modestly, from 88.5% to 90.1%.
2. To optimize specificity, we tested the effect of treating
all red flags as absolute exclusions for probable PD
(leaving supportive criteria irrelevant). Doing this,
the false-positive rate lowered from 11.5% to 4.7%
(still higher than the clinically established false-
positive rate of 1.6%). However, sensitivity dropped
below the target 80% threshold (ie, 79.3%). Alterna-
tively, removing all the red flags (ie, using only a sim-
ple criterion with the 8 absolute exclusions) would
provide poor specificity (ie, 64%).
3. To enhance the accuracy of PD diagnosis in early
PD (ie, to develop a high-certainty early-PD category
for future clinical trials), we examined this group in
more detail. For short-duration disease or untreated
PD, it is very difficult to meet criteria for clinically
established PD criteria (eg, dyskinesia and levodopa
response often do not apply), leaving no clear high-
specificity option for early PD. Therefore, we ana-
lyzed the effect of treating red flags as absolute
exclusion criteria (and removing duration compo-
nents) in cases with disease duration < 5 years. This
yielded 95.4% specificity and 68.9% sensitivity
compared with the gold standard, clinical diagnosis
(by comparison, the existing clinically established
category in this group had 98.7% specificity and
47.1% sensitivity).
Discussion
The MDS clinical diagnostic criteria for PD were
designed to mimic an expert clinician’s diagnostic pro-
cess and to codify and standardize diagnosis for use in
clinical research and for clinical diagnosis by those with
less expertise in PD. This study was designed to test how
well this codification was done. We found an overall
accuracy/concordance rate of 92.4%, with sensitivity of
94.5% and specificity of 88.5%. The UK Brain Bank cri-
teria had a discordance/error rate approximately double
the MDS criteria, and both sensitivity and specificity
were lower than with MDS criteria. Accuracy/concor-
dance was higher in those who were younger and who
had longer disease duration.
Although not the primary purpose of this study, our
study does provide important information on the util-
ity of olfactory testing in PD. Most studies have sug-
gested that olfactory testing has approximately 80%
sensitivity and >80% specificity for the diagnosis of
PD.10
We found much lower diagnostic performance
in this very large and systematically assessed sample. It
should be noted that the shorter versions of olfactory
tests were used, and it is possible that longer tests (eg,
the 40-item University of Pennsylvania Smell Identifi-
cation test) would perform better. However, many
other previous studies also used shorter versions. It is
possible that cultural factors from exposure to differ-
ent odors may be important; for example, the sensitiv-
ity of olfactory testing was lower in Beijing (49%)
than in the other centers (70%). However, specificity
did not substantially differ according to site (eg, 59%
in Beijing vs 65% in the other centers), and specificity
was <80% in every single center. Therefore, olfactory
testing’s diagnostic utility remains unclear.
Why did both the sensitivity and specificity of the
MDS criteria exceed UK Brain Bank criteria? For speci-
ficity, no clear pattern was detected. Of 27 false-
positives caught by MDS criteria (UK Brain Bank posi-
tive for PD, but MDS criteria and gold standard nega-
tive), 14 (52%) were excluded for absolute exclusions
(commonest: cortical sensory loss [5], absent levodopa
response [5]), and 13 for unbalanced red flags (com-
monest red flags overall: frequent falls [15], bilateral
symmetric parkinsonism [8]). However, regarding the
improved sensitivity, most related to clear advances in
the field. Of 37 false-negative diagnoses detected by
MDS criteria, 12 (32%) were excluded for > 1 affected
family member (underscoring advances in the genetics
of PD), 5 (14%) for head injury (now well recognized
as a PD risk factor11
), 4 (11%) for early autonomic
involvement (now recognized as a common prodromal
marker12
), 2 (5%) for dementia (now recognized in
early PD13
), and 1 (3%) for neuroleptic exposure
(reflecting the development of atypical neuroleptics
and/or increasing recognition that drug-induced parkin-
sonism is a potential prodromal marker of true PD14
).
This underscores the need to revise the MDS criteria
periodically in the future to reflect advances in our
understanding of PD. This will become especially criti-
cal if a reliable diagnostic biomarker (eg, neuroimaging,
tissue diagnosis, blood/cerebrospinal markers) becomes
firmly established.
Overall, the sensitivity and specificity of probable pro-
dromal PD clearly exceeded the 80% target threshold.
Based on our subanalysis, should the MDS criteria be
modified? Because sensitivity exceeded specificity, tweak-
ing criteria might help to balance these slightly (eg,
6 Movement Disorders, Vol. 00, No. 00, 2018
P O S T U M A E T A L](https://image.slidesharecdn.com/postuma2018-190801223829/85/Postuma2018-6-320.jpg)

