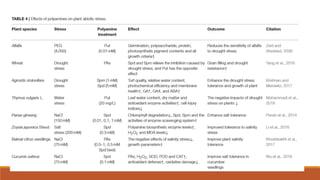
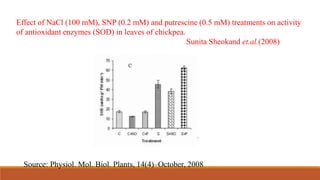
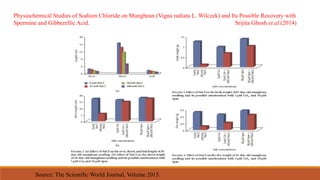
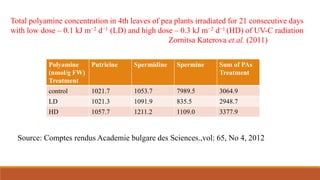
This document discusses polyamine biosynthesis and its role in modulating plant development and stress response. It outlines that polyamines such as putrescine, spermidine, and spermine are involved in fundamental cell processes and help plants withstand stress. The document summarizes polyamine biosynthesis pathways, occurrence in plants, physiological effects, and role in oxidative stress response and tolerance to stresses like drought, heat, cold, and salinity. It also reviews evidence from research studies on the effects of polyamines and inhibitors on antioxidant enzyme activity and plant tolerance under stress conditions.