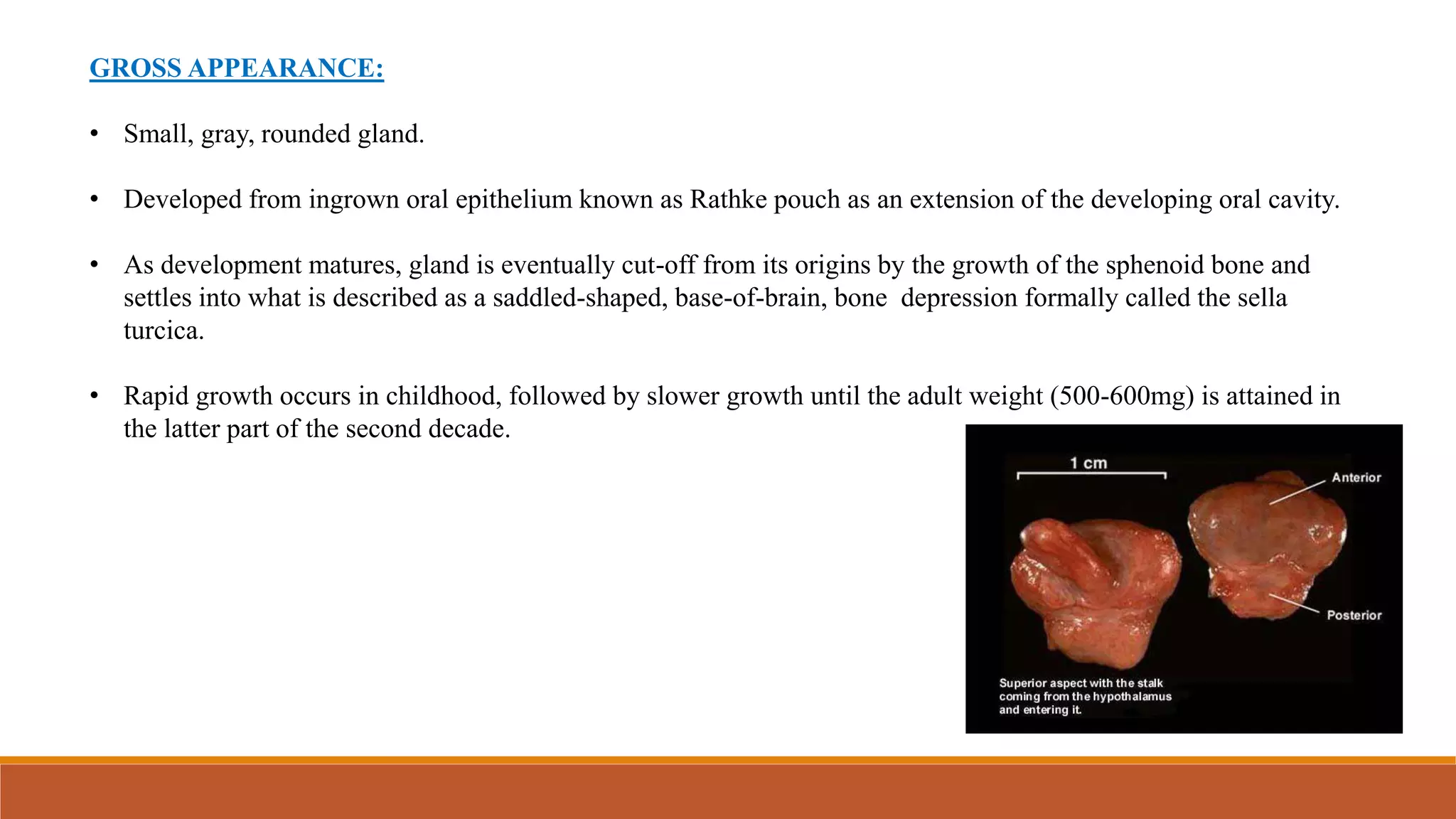
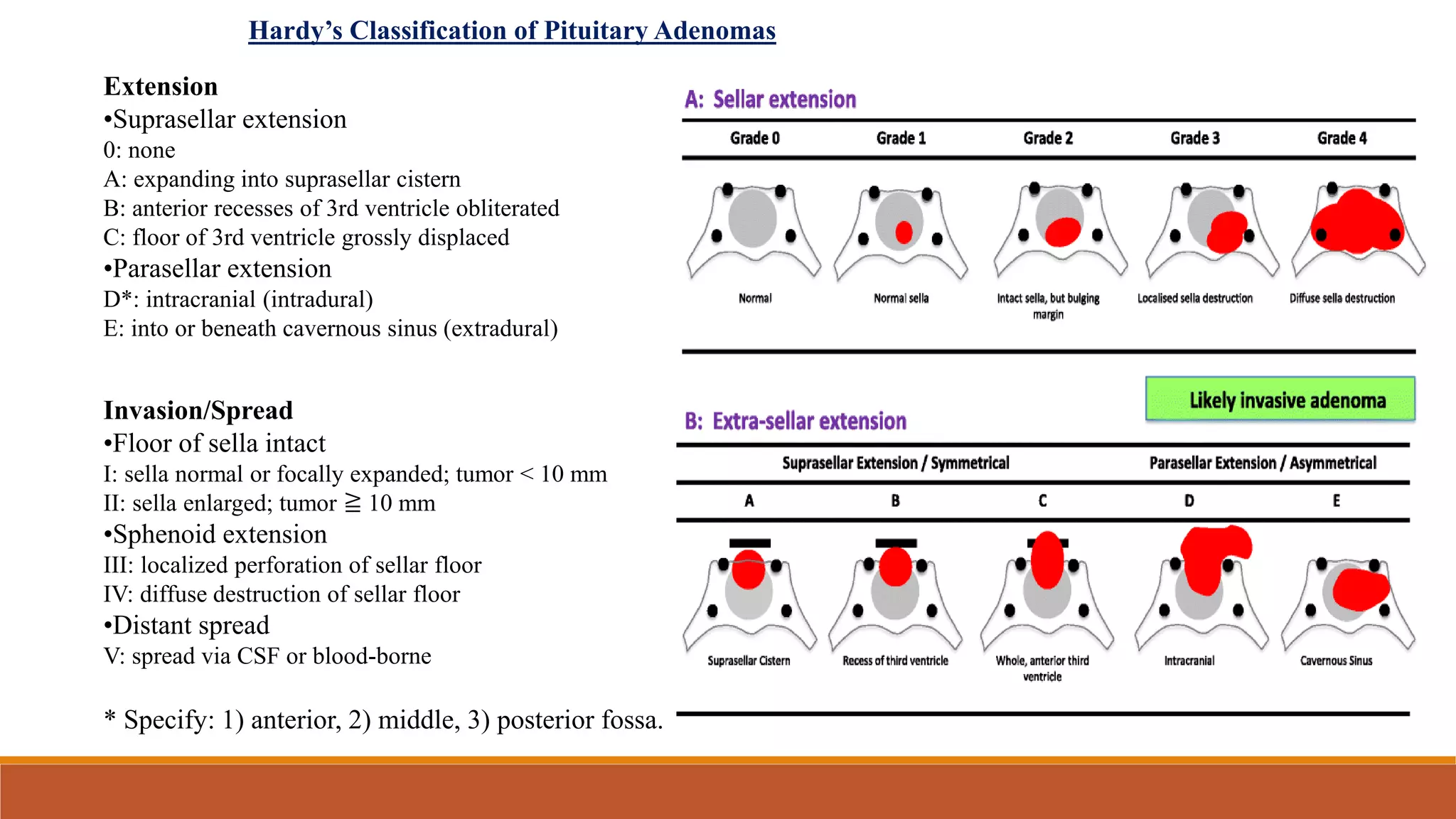
The document discusses the anatomy, physiology, incidence, and management of pituitary tumors, primarily focusing on pituitary adenomas, which are the most common type of pituitary tumor. It details the classification, diagnostic workup, treatment options including surgery, medical management, and radiotherapy, while emphasizing the importance of achieving hormone normalization and minimizing complications. The document concludes that stereotactic irradiation methods effectively control tumor growth and improve patient outcomes, although hypopituitarism remains a common late complication.











![CLASSIFICATION:
PITUITARY ADENOMAS
Non-Functioning PA [NFPA]
Functioning PA [FPA] Macro adenomas [ ≥10mm]
Micro adenomas [<10mm]
• Prolactinomas comprises 40-57% of all adenomas, followed by NFPA (28-37%), GH secreting adenomas (11-
13%) and ACTH secreting adenomas (1-2%).
• Pituitary adenomas that secrete FSH, LH, TSH are rare.
Pico adenomas [<3mm]
Giant adenomas [>4cm]](https://image.slidesharecdn.com/pituitarytumors-230814131247-369a3255/75/PITUITARY-TUMORS-pptx-13-2048.jpg)




















![CONVENTIONAL RT:
o 2 Fields technique:
• by opening a 5x5 field with centre at pituitary
• Surface marking: 2.5cm anterior and superior to external auditory meatus [point of base of sella turcica/middle of
pituitary]
• Treatment volume will include pituitary gland with margin.
• Problem: high dose to temporal lobe](https://image.slidesharecdn.com/pituitarytumors-230814131247-369a3255/75/PITUITARY-TUMORS-pptx-35-2048.jpg)





