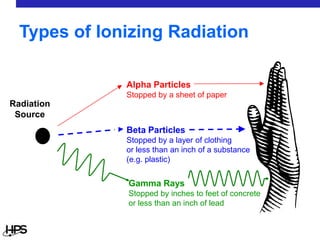
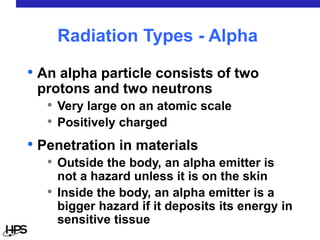
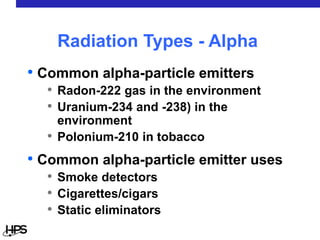
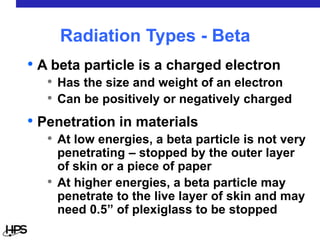
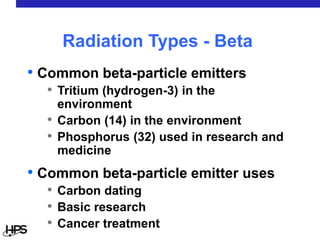
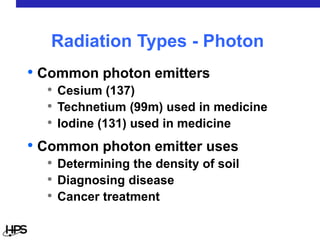
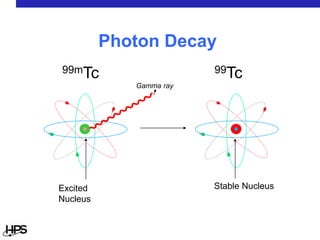
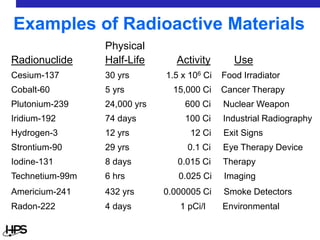
Radiation comes in three main types: alpha particles, beta particles, and gamma rays. Alpha particles are large and positively charged, but do not penetrate far, stopped by skin or paper. Beta particles are electrons that can penetrate further, stopped by clothing or plastic. Gamma rays have no charge or mass and penetrate the deepest, requiring thick concrete or lead to stop them. Each type interacts differently with matter and can be sources of radiation both inside and outside the body from uses like medical imaging, smoke detectors, and environmental sources like radon.