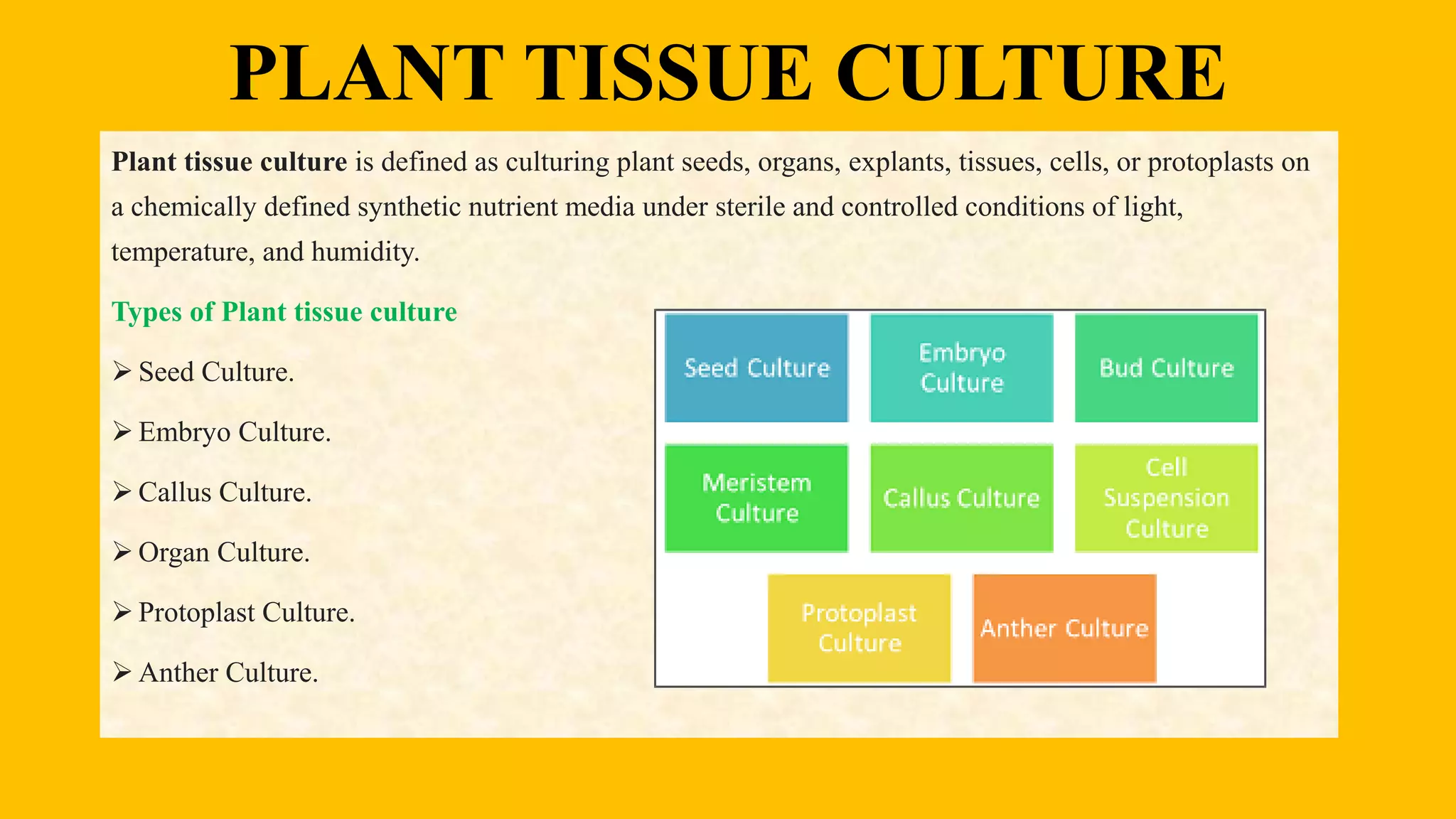
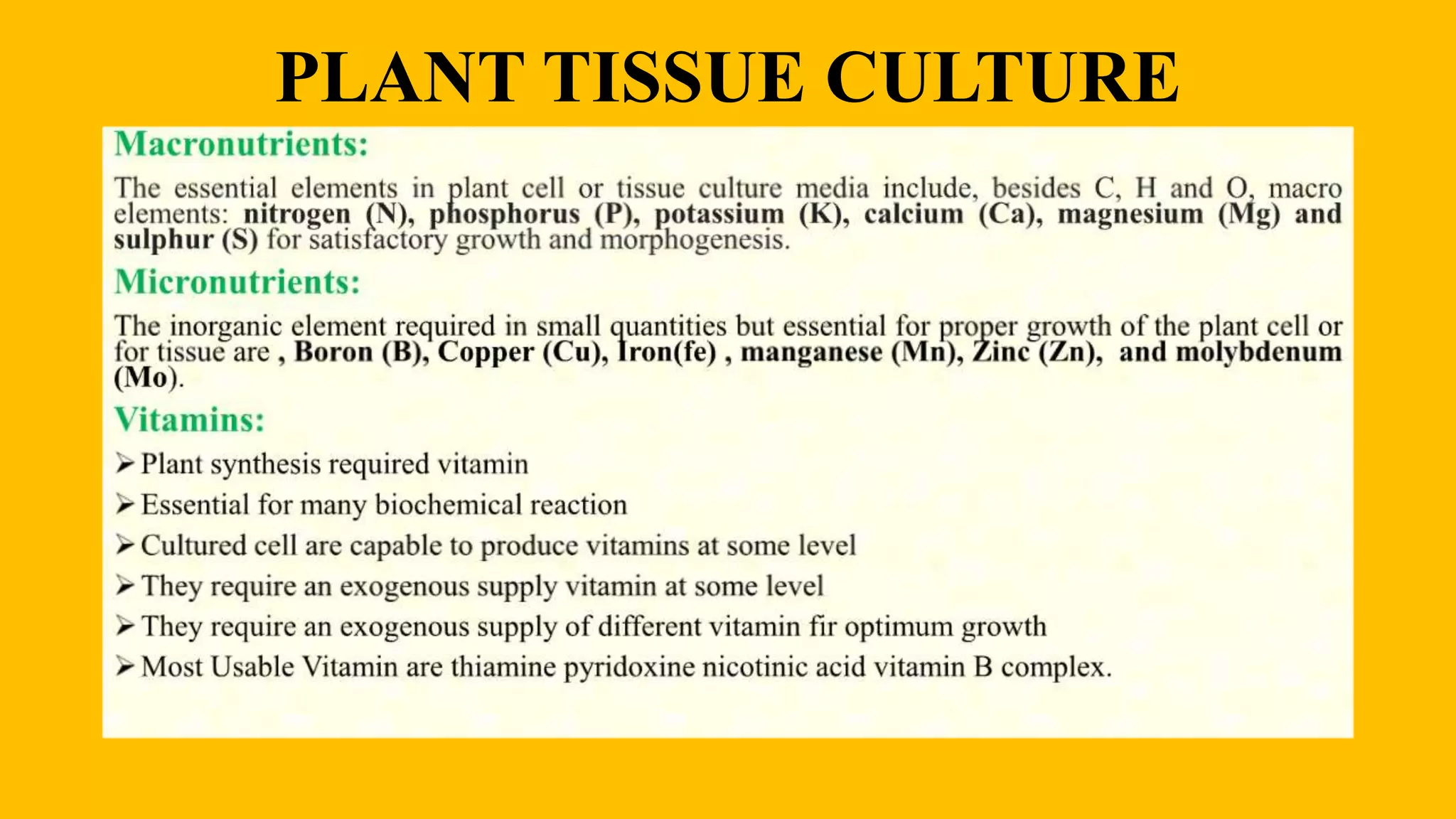
This document discusses plant tissue culture, including the types, steps involved, and procedures. It describes the different types of plant tissue culture such as seed culture, embryo culture, and anther culture. The key steps are initiation, multiplication, root formation, shoot formation, and acclimatization. The procedures covered are sterilization of materials, preparation and sterilization of explants, production and proliferation of callus, subculturing, and suspension culture. The document provides details on the composition of culture media and the roles of macronutrients, micronutrients, vitamins, nitrogen supplements, carbon sources, growth regulators, and solidifying agents.