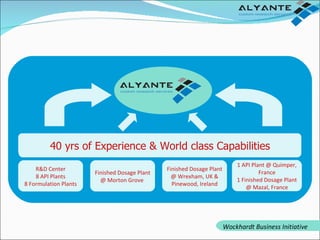
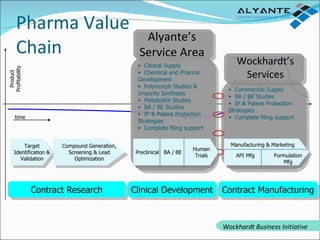
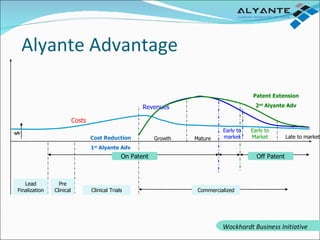
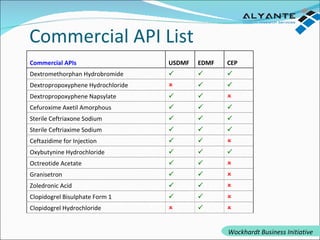
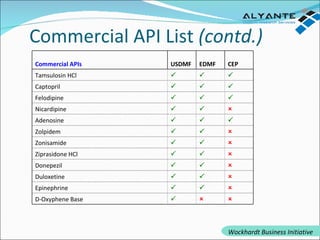
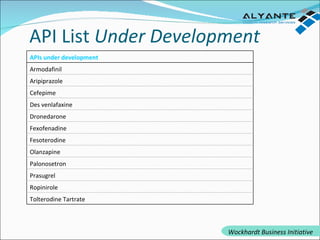
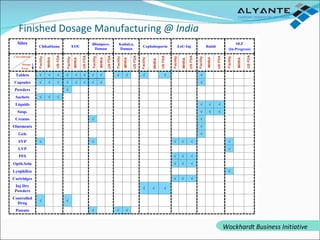
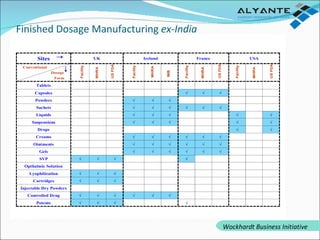
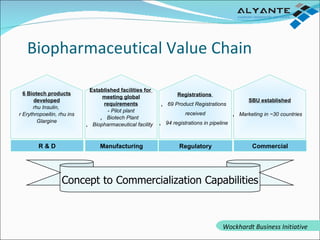
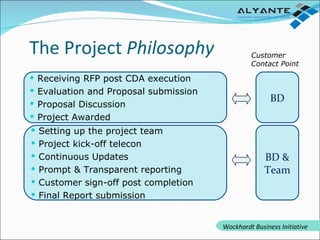
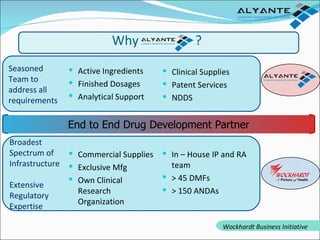
Wockhardt is a global pharmaceutical company offering comprehensive services in drug development, including contract research, manufacturing, and marketing across various dosage forms. They emphasize innovation through advanced capabilities in API manufacturing, regulatory support, and analytical excellence, catering to diverse markets worldwide. Their initiative, Alyante, focuses on delivering tailored solutions and maintaining strong partnerships, ensuring timely and high-quality outcomes in pharmaceutical development.