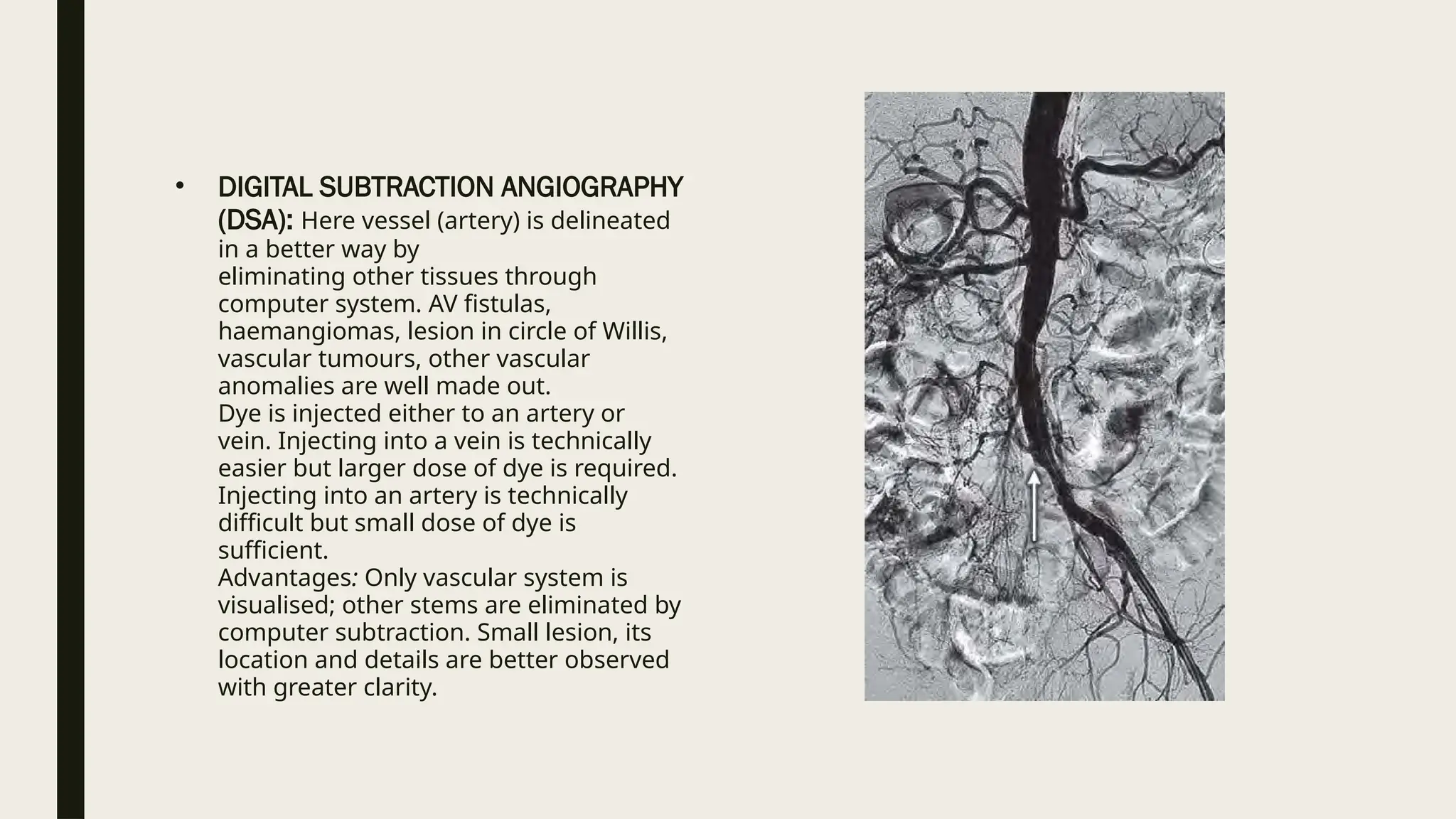
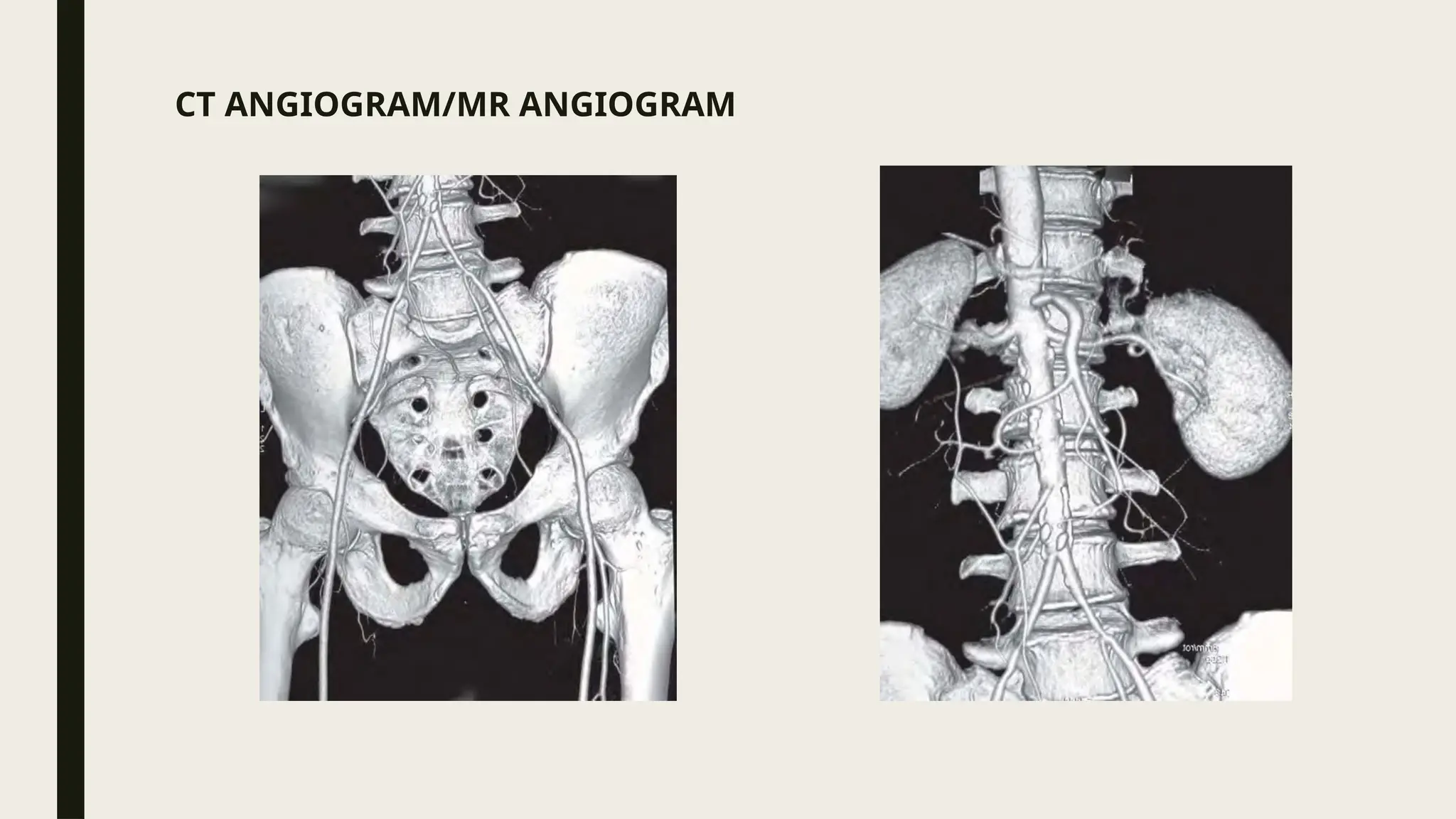
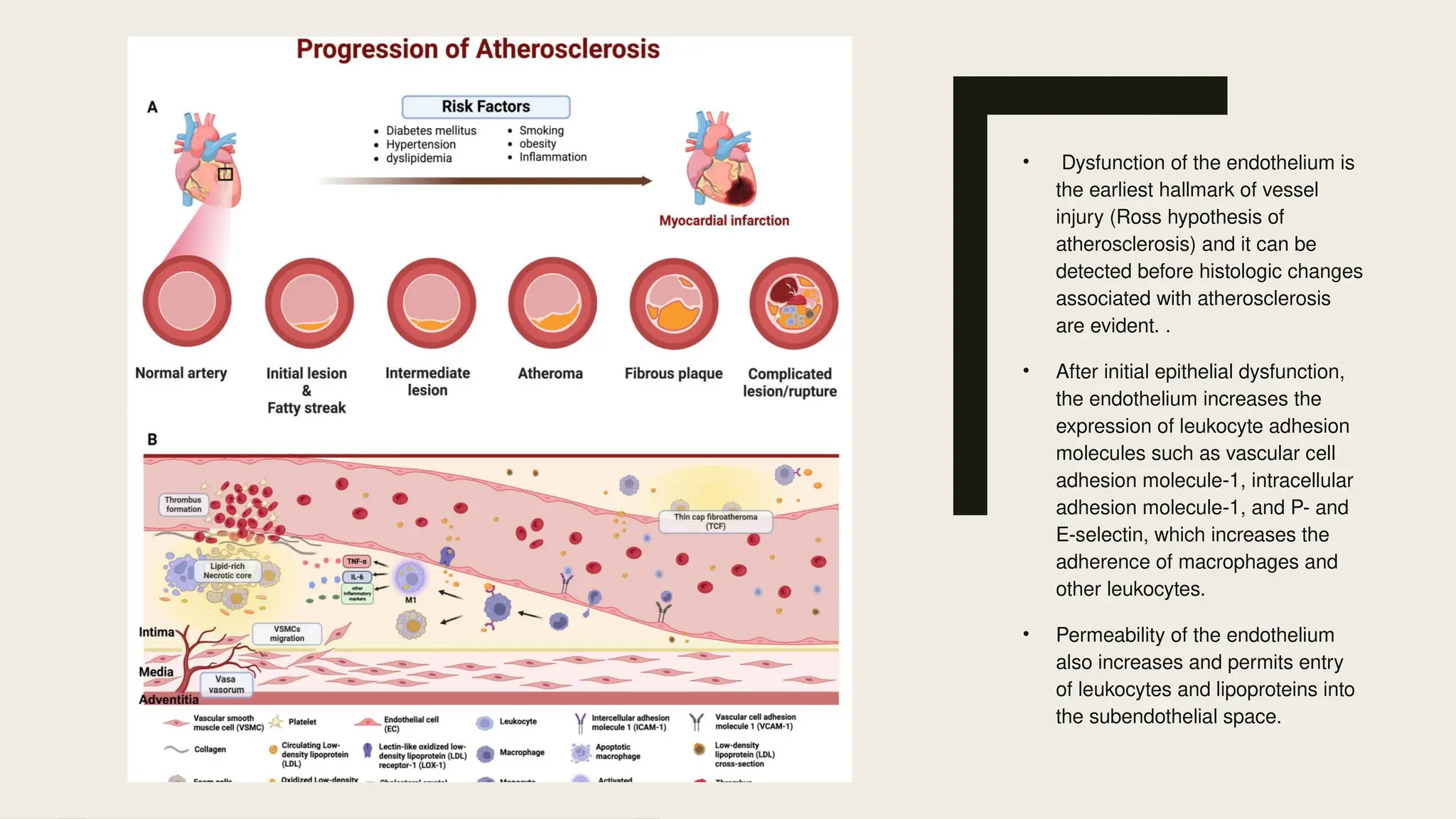
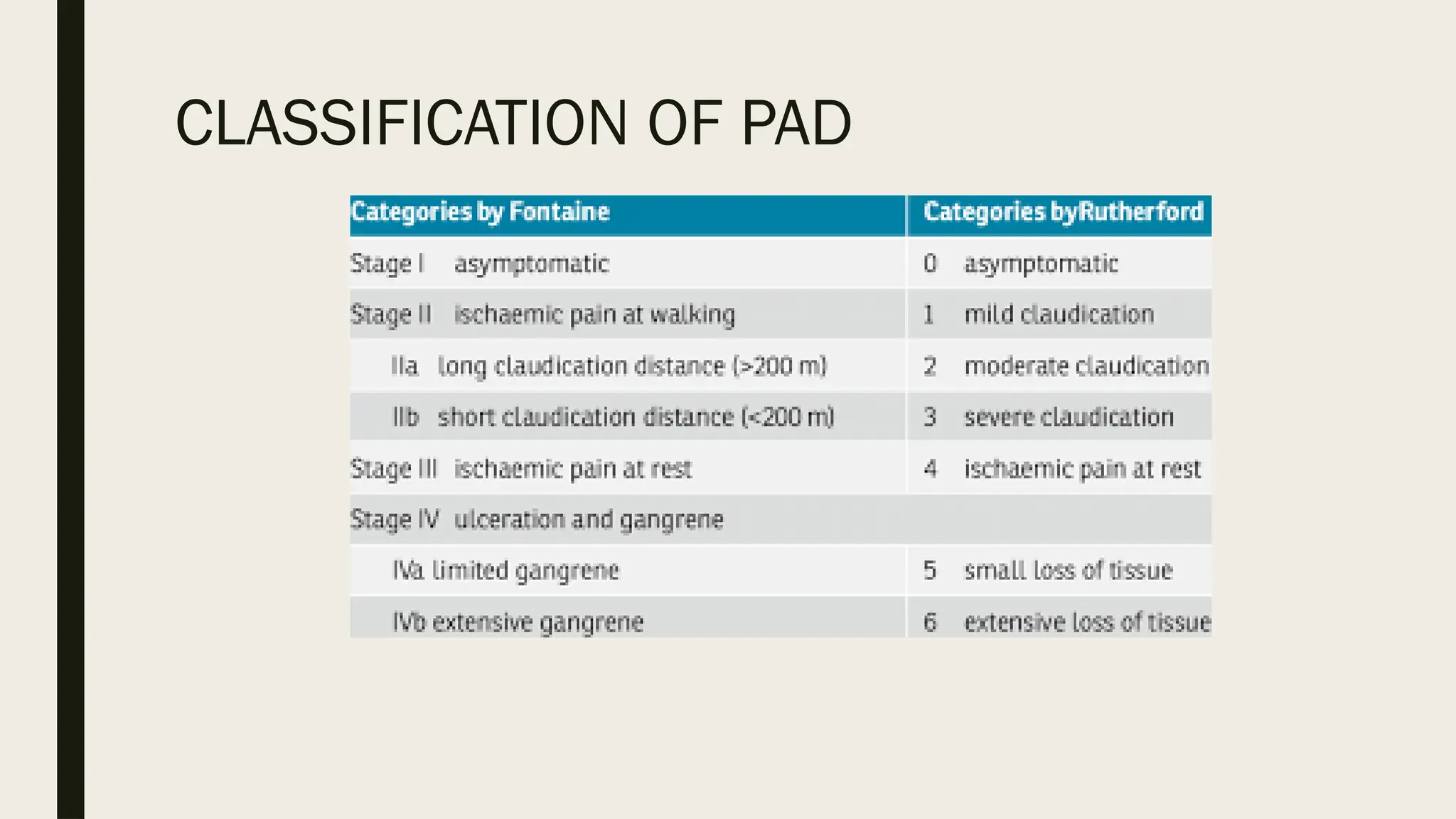
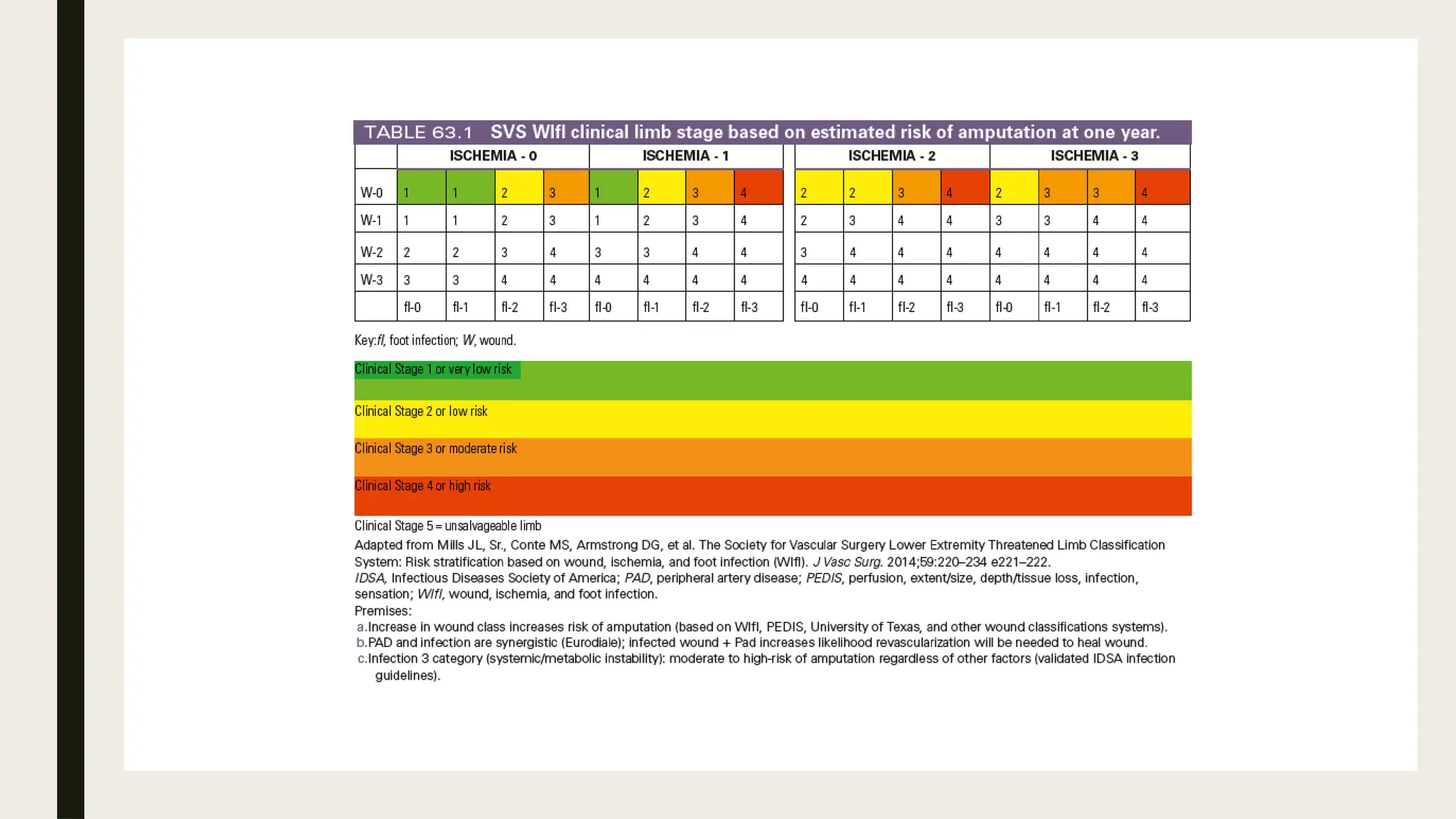
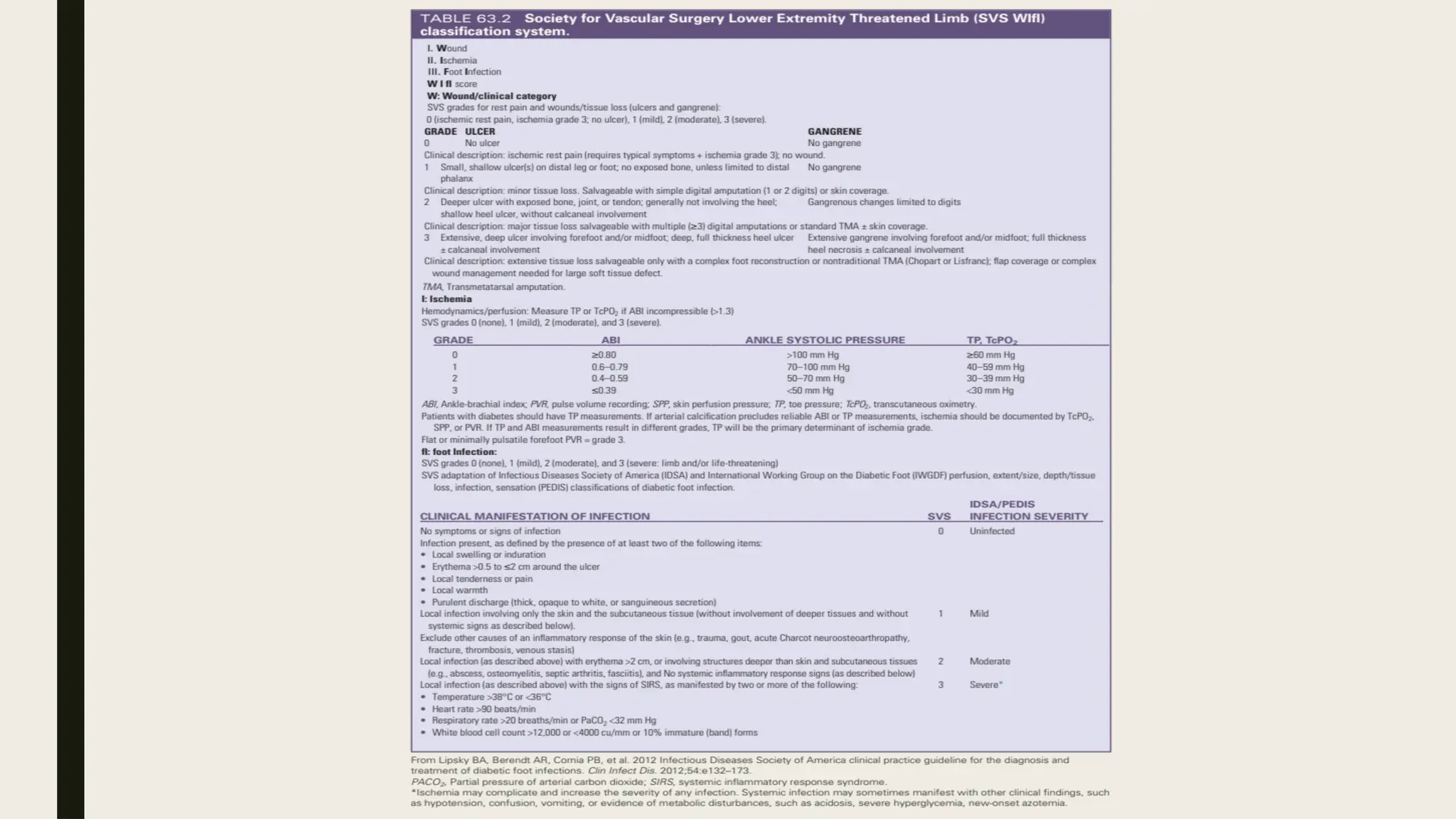
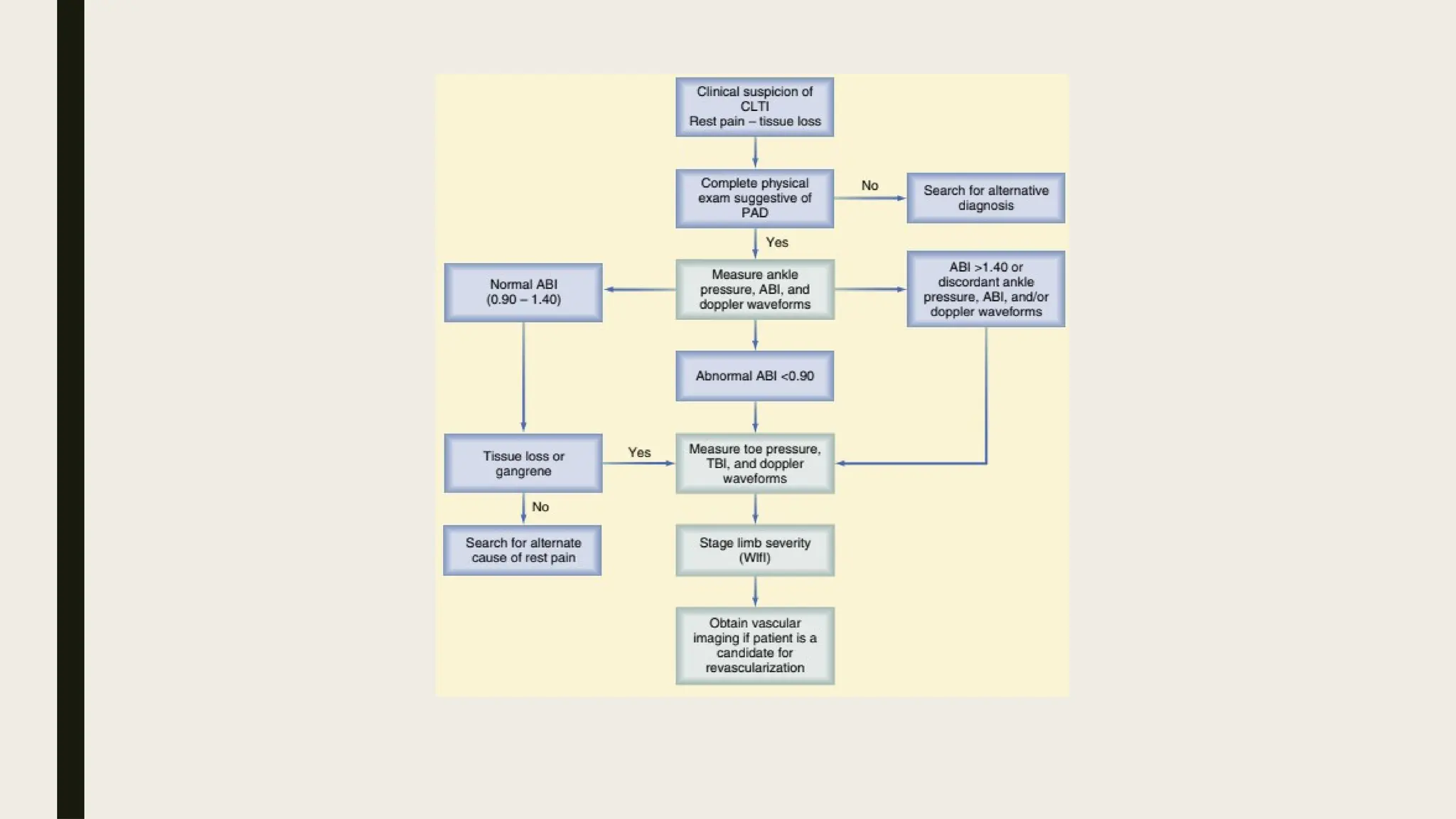
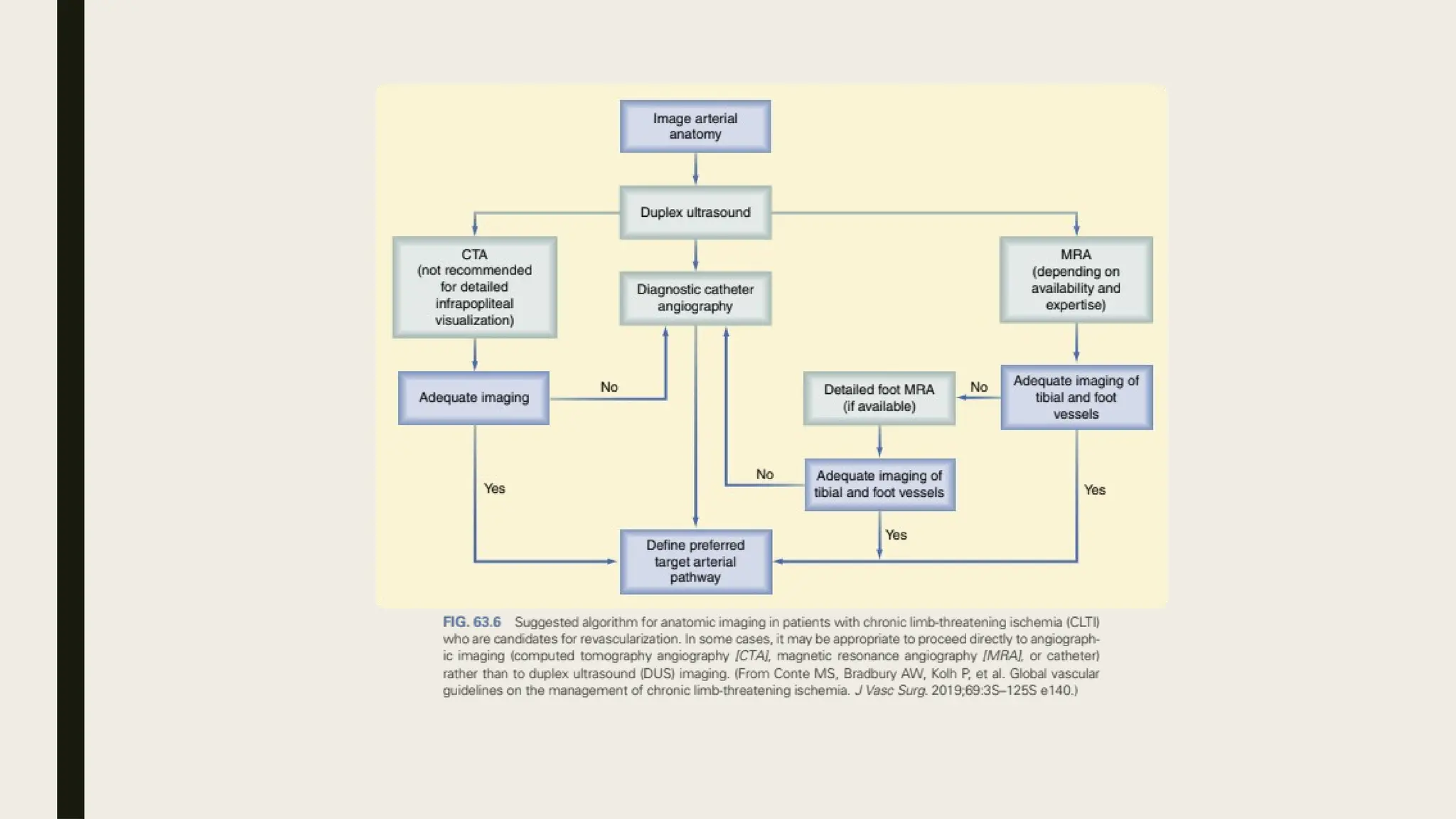
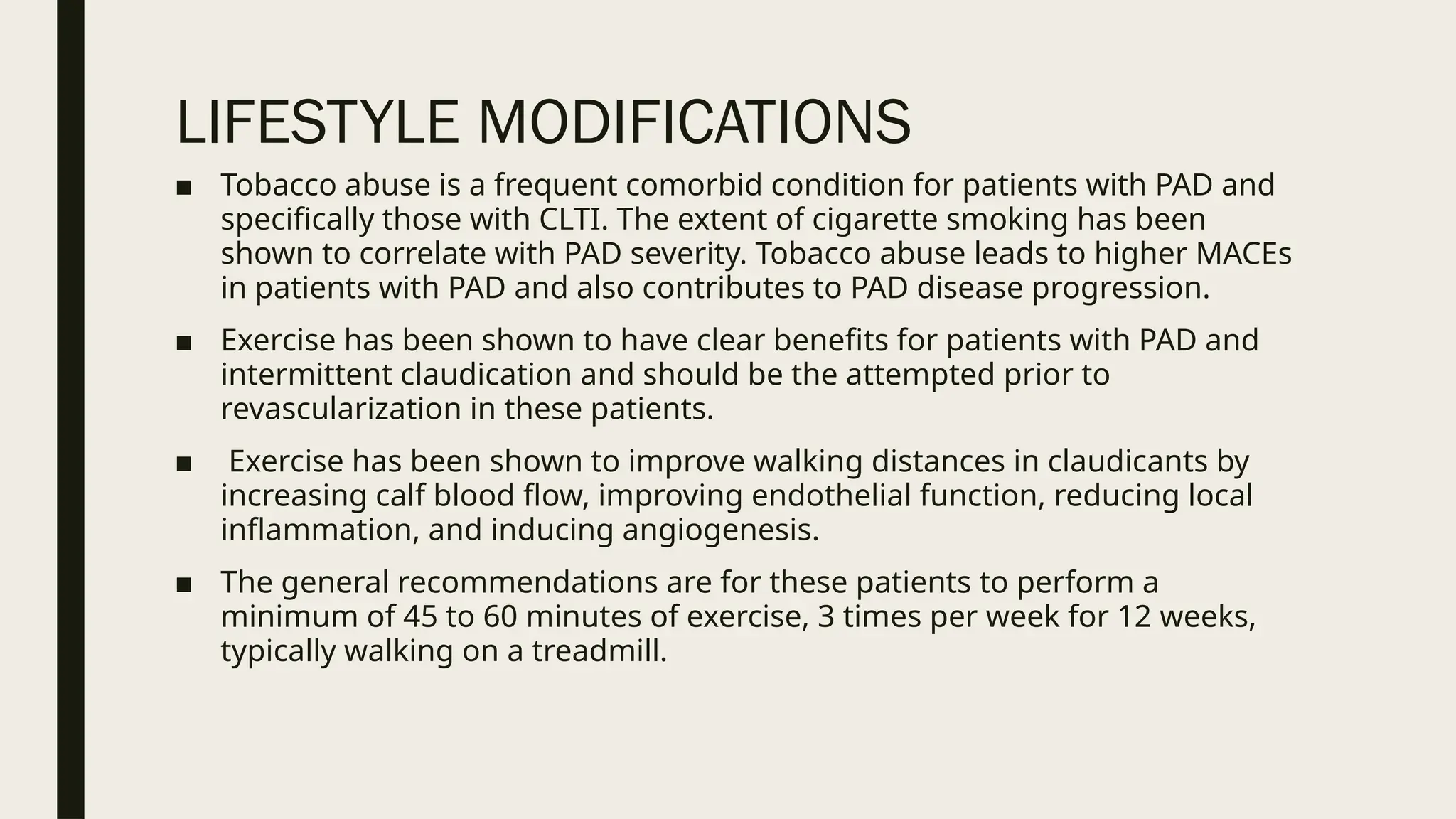
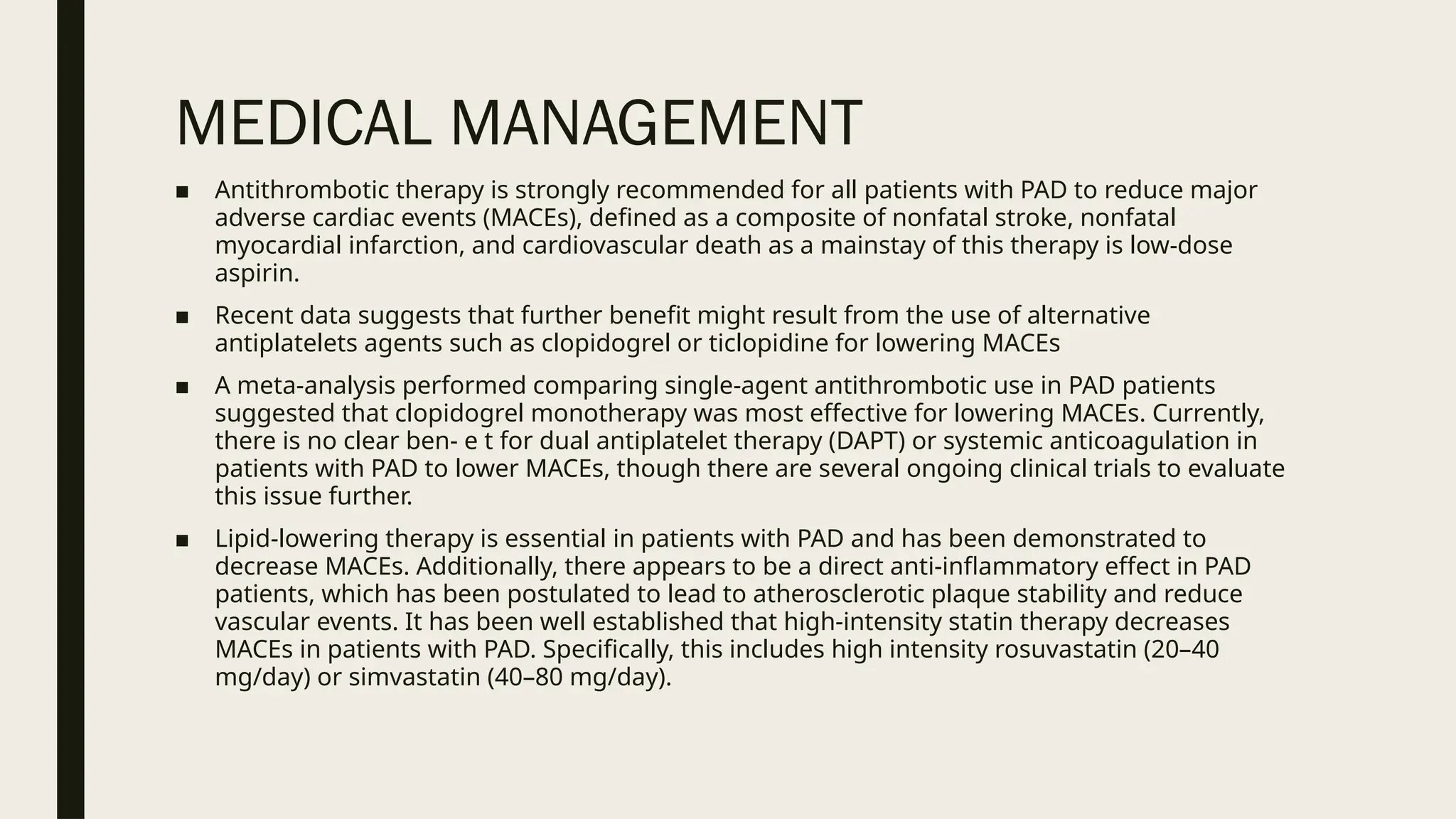
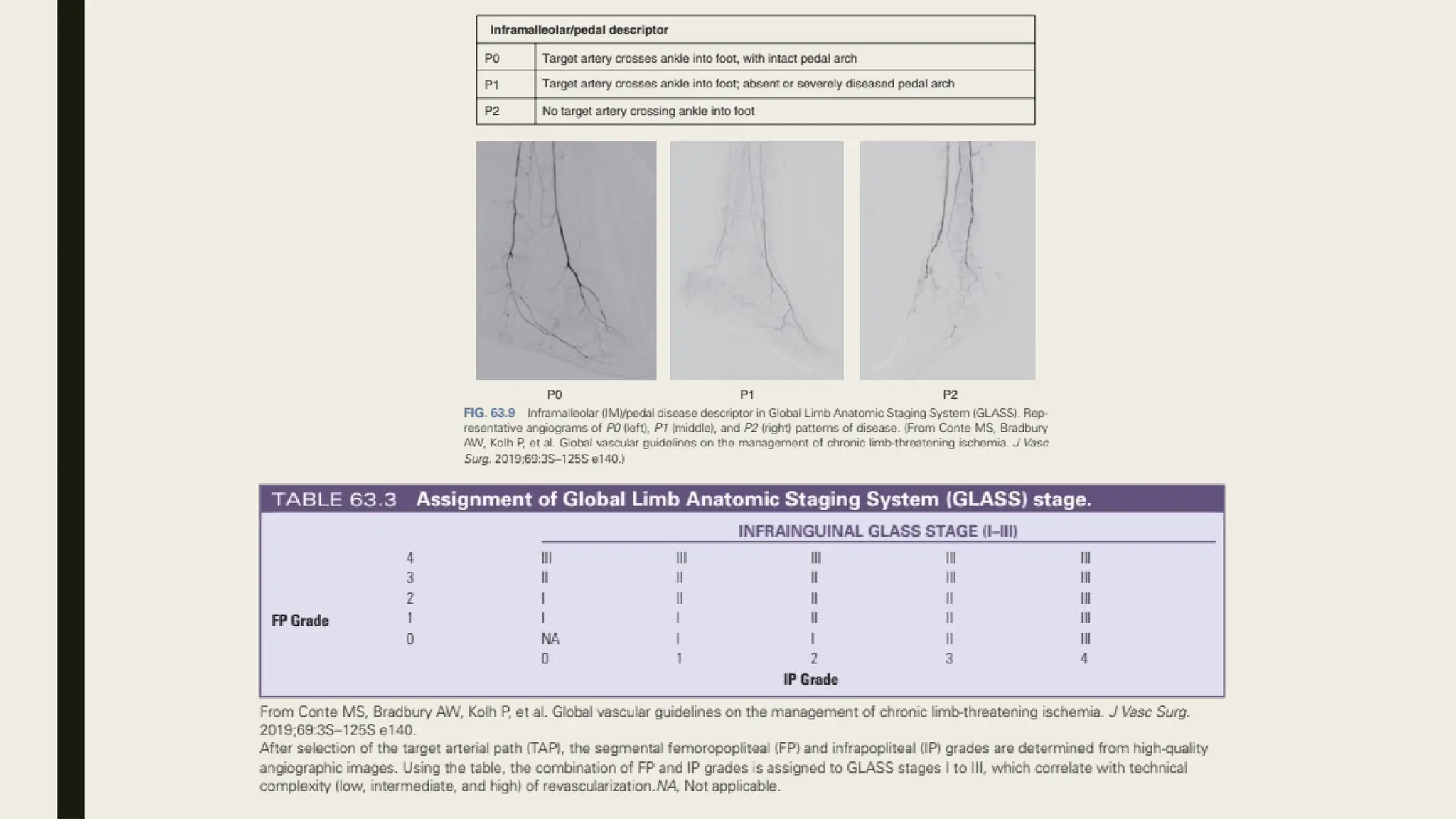
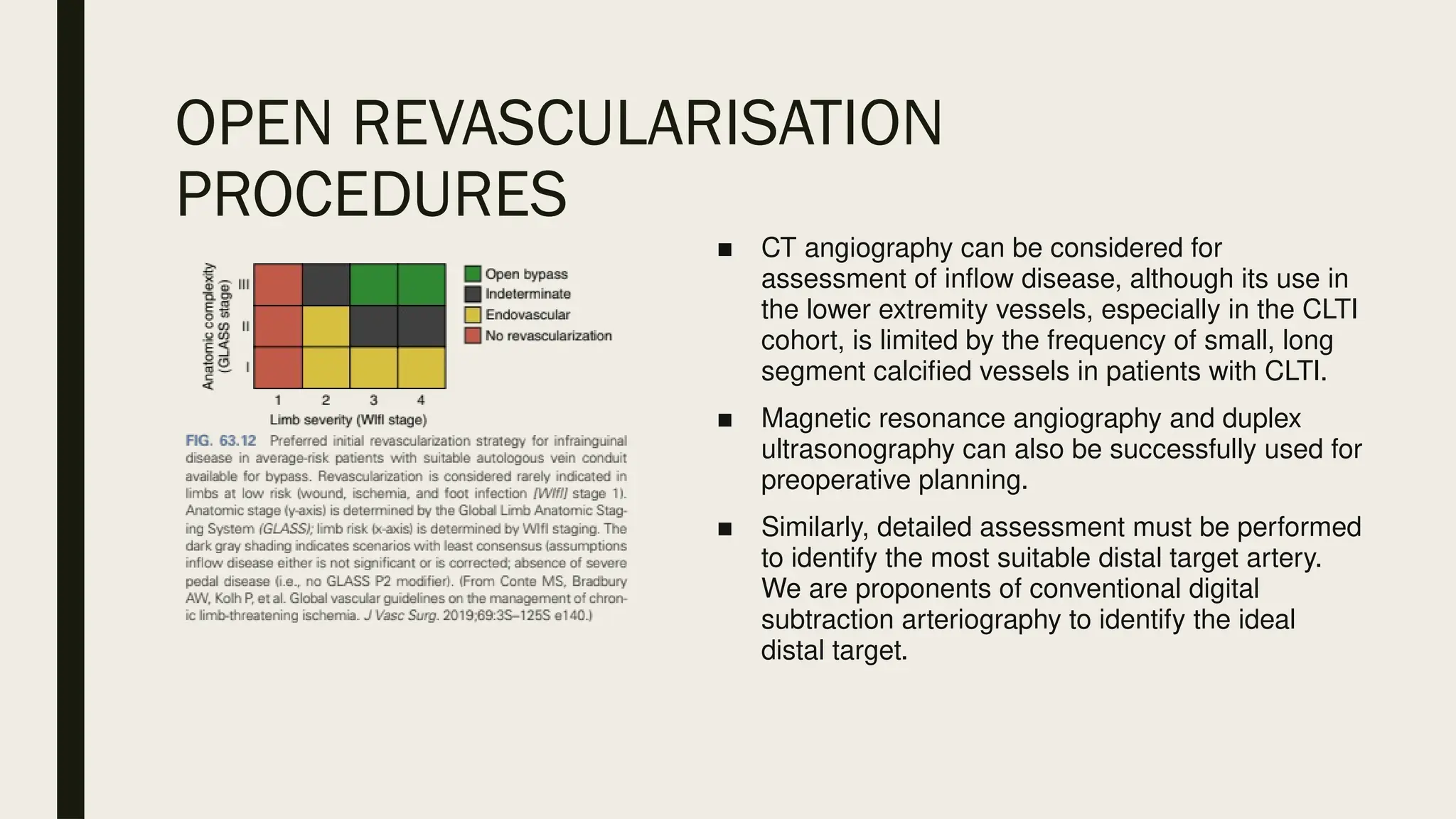
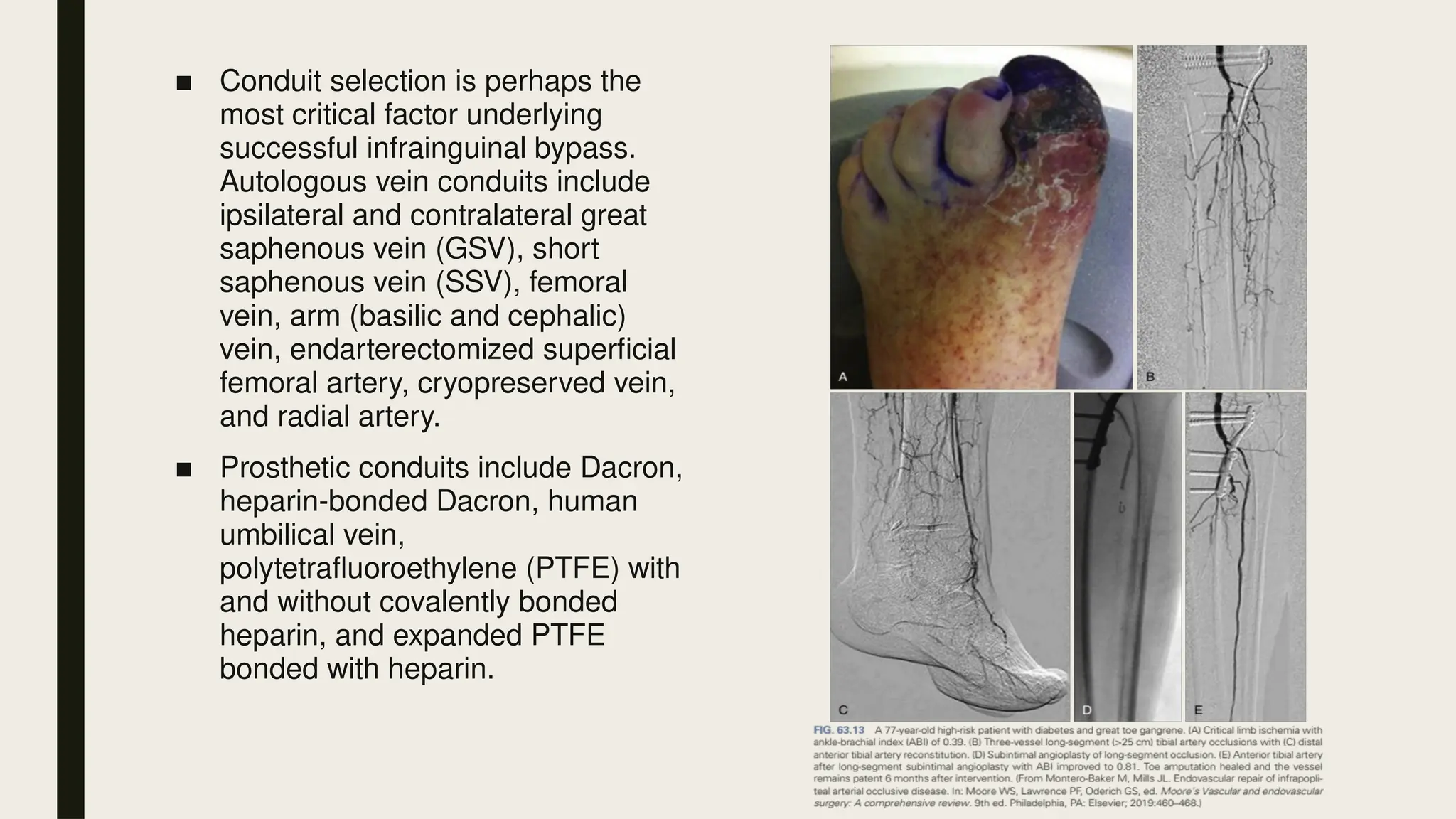
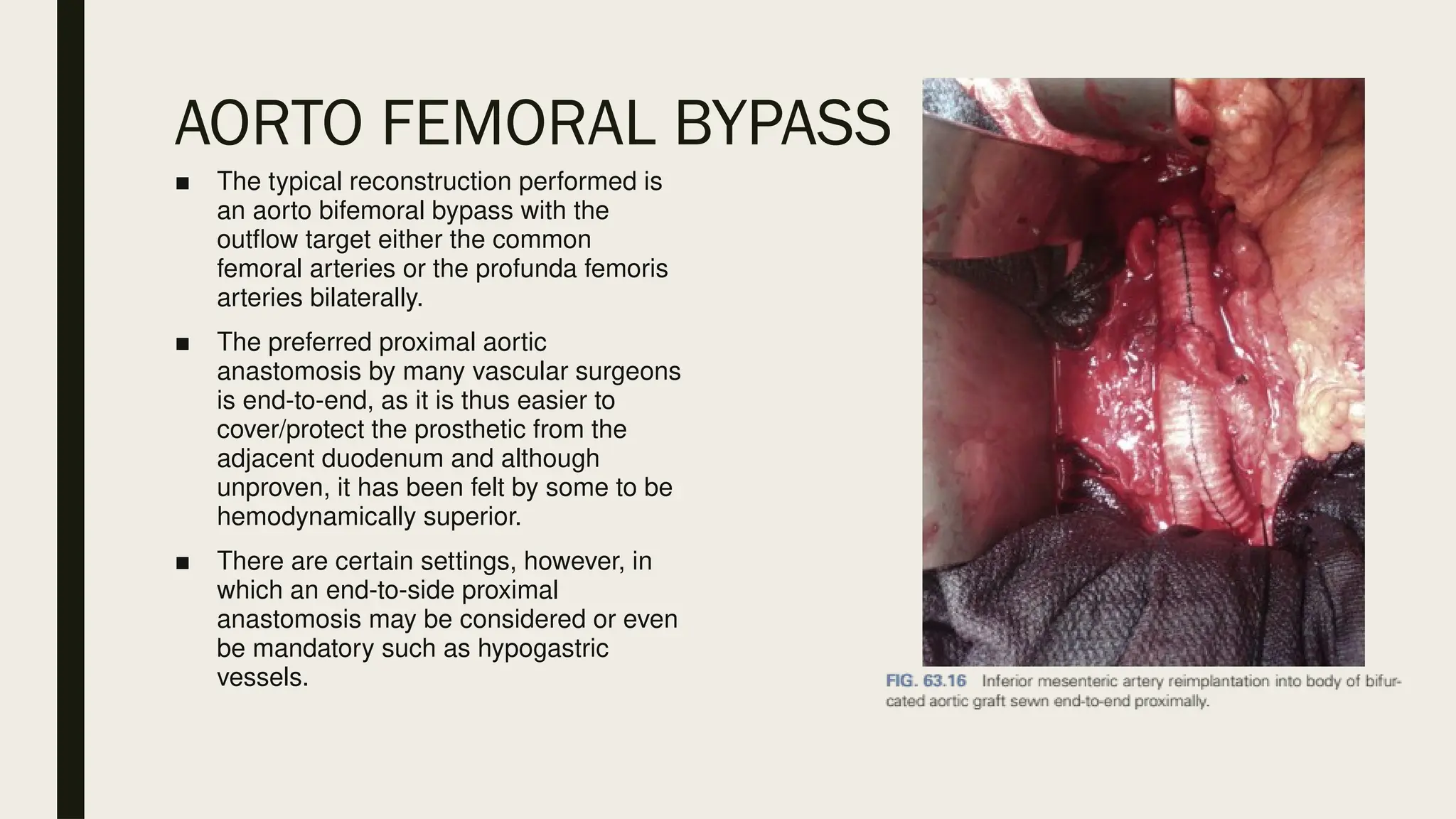
Peripheral arterial disease (PAD) is a progressive blood vessel disorder characterized by narrowing or blockage, affecting circulation, particularly in the legs, leading to symptoms such as leg cramping and ulcers. Key risk factors include age, smoking, diabetes, and cardiovascular history, with complications like limb amputation and stroke. Evaluation involves assessing symptoms and risk factors, along with diagnostic tests like the ankle-brachial index and doppler studies to determine the disease's severity and location.