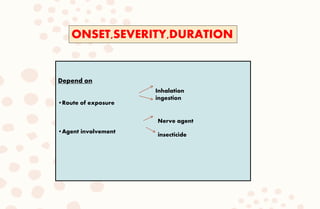
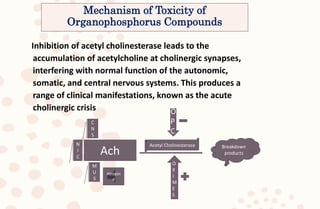
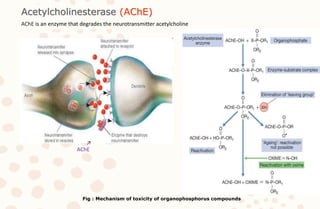
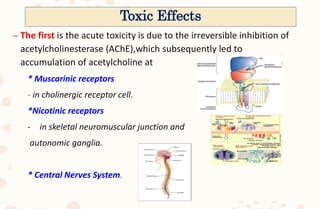
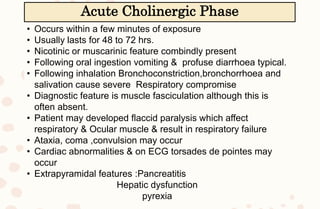
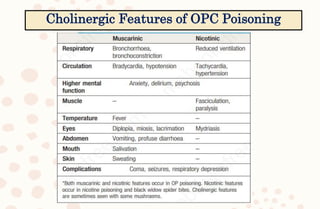
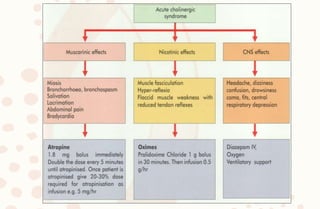
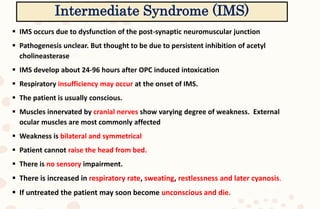
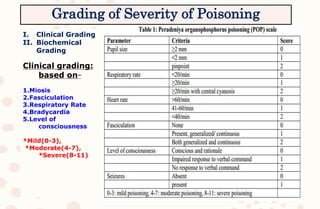
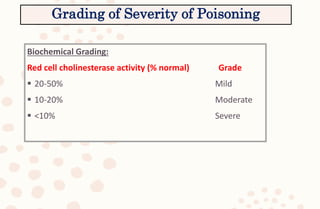
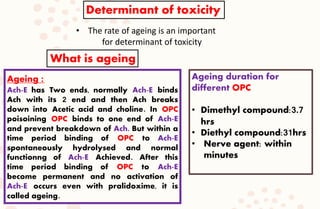
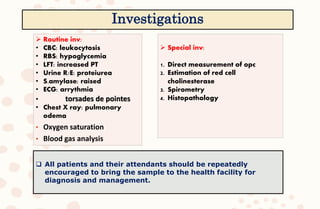
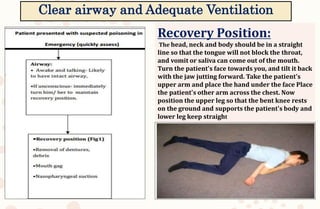
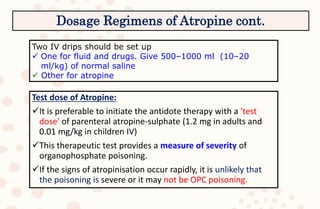
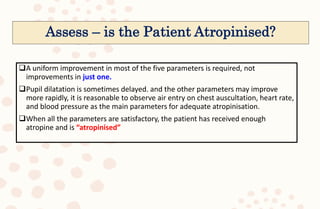
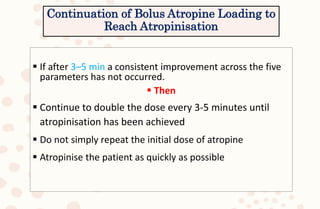
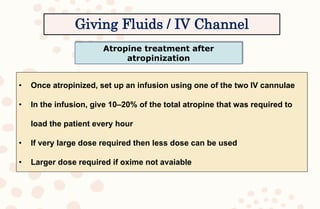
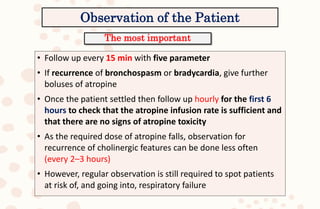
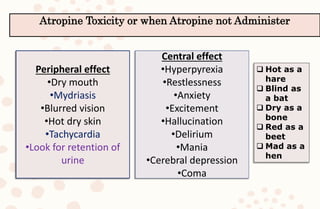
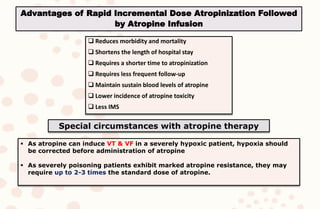
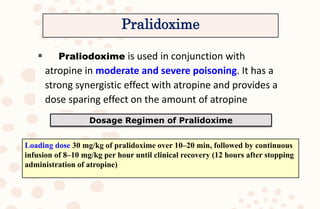
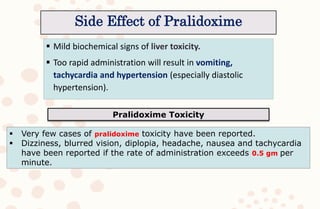
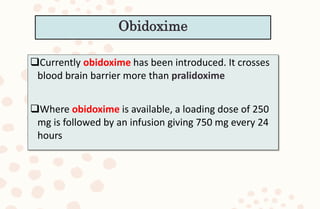
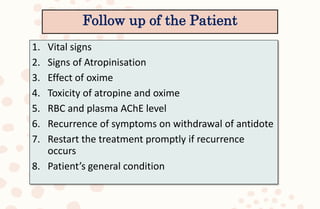
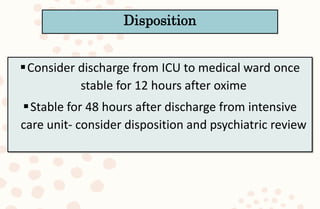
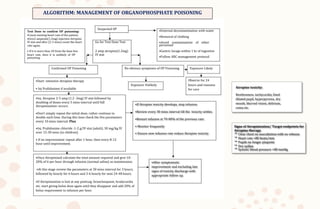
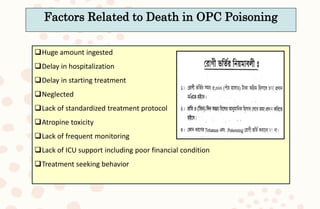
The document discusses organophosphorus compound (OPC) poisoning, including what OPCs are, their various uses, mechanisms of toxicity, clinical manifestations, grading of severity, investigations, management with atropinization and oxime therapy, and dosage regimens for atropine treatment.