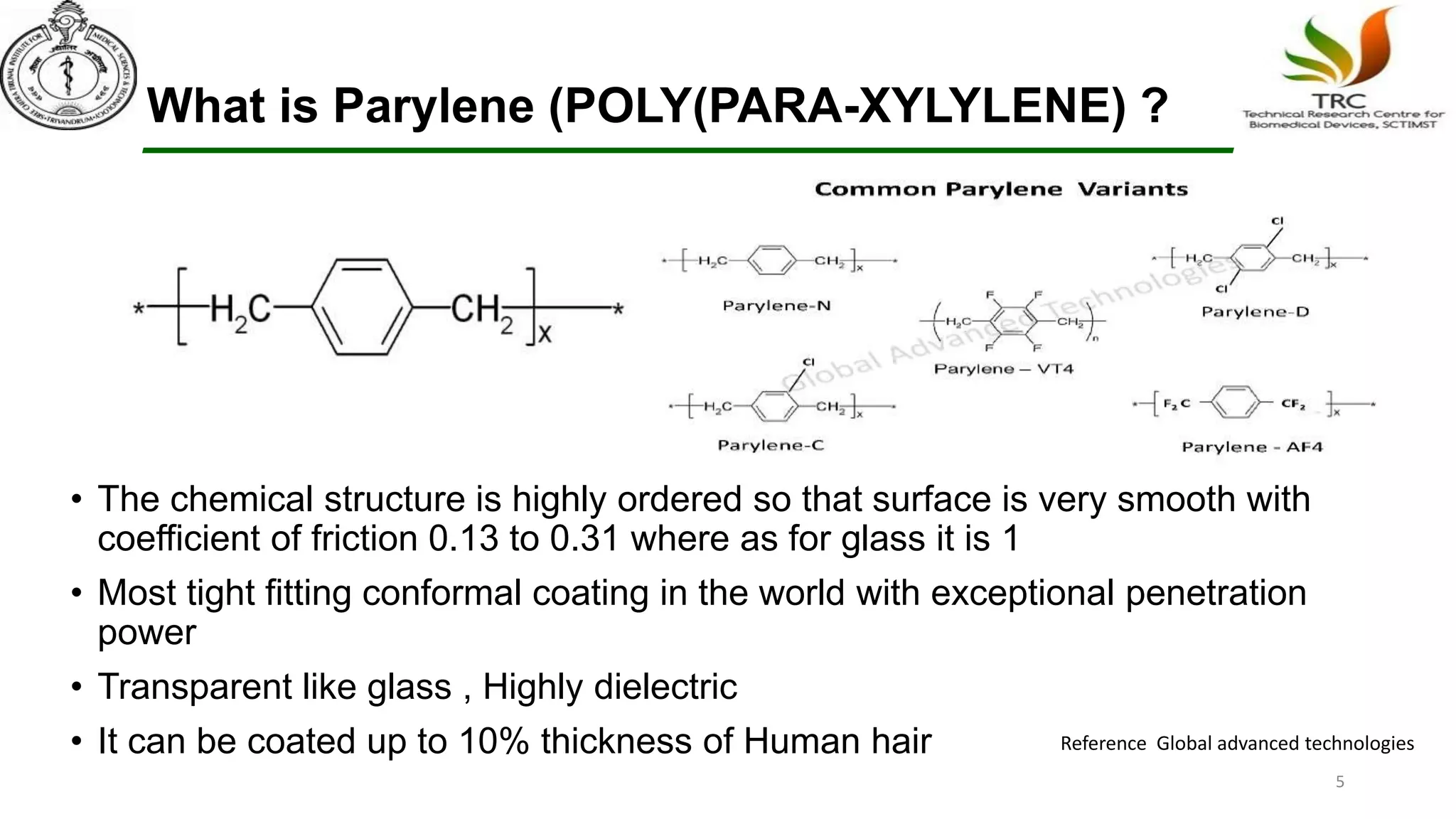
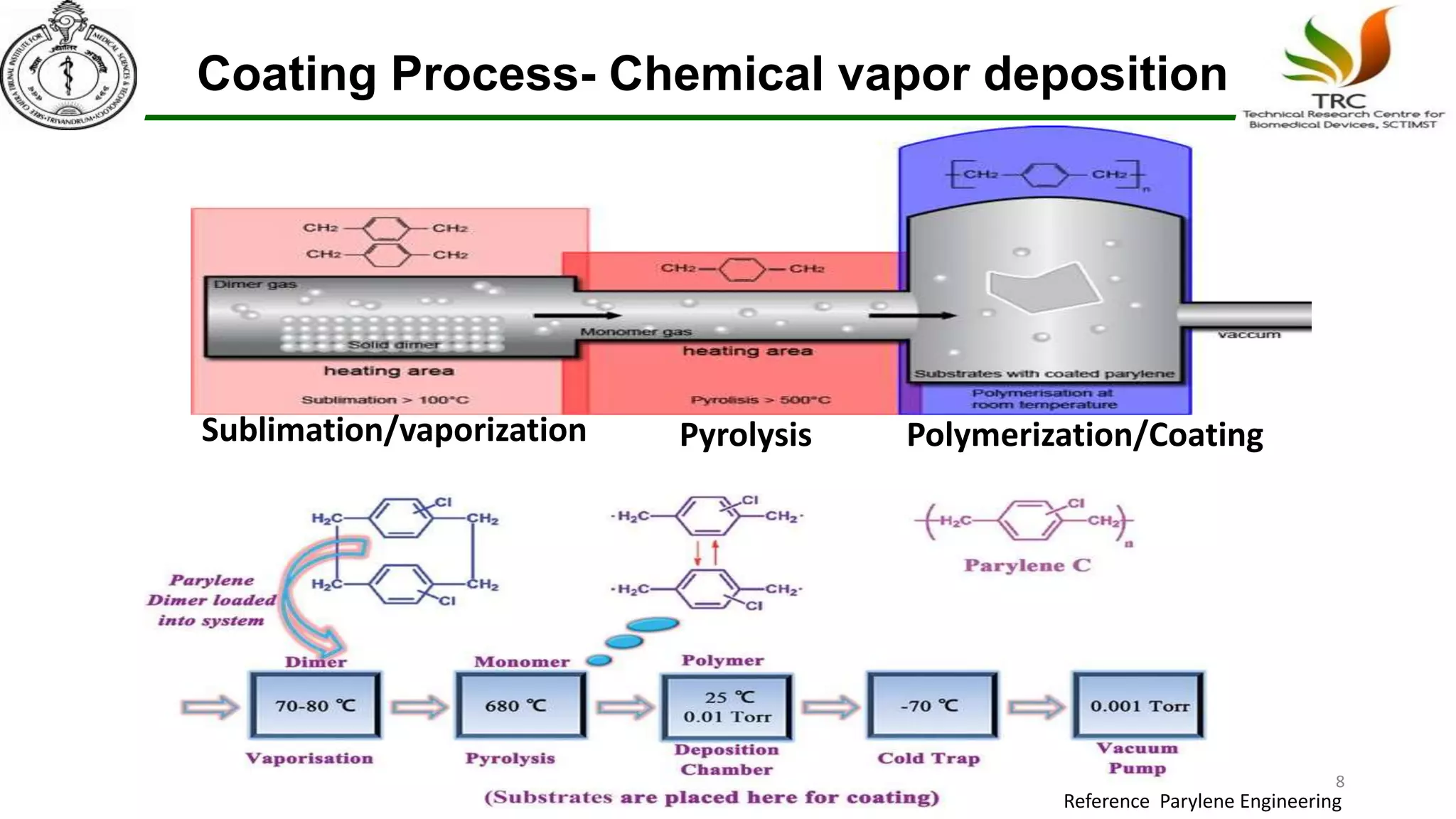
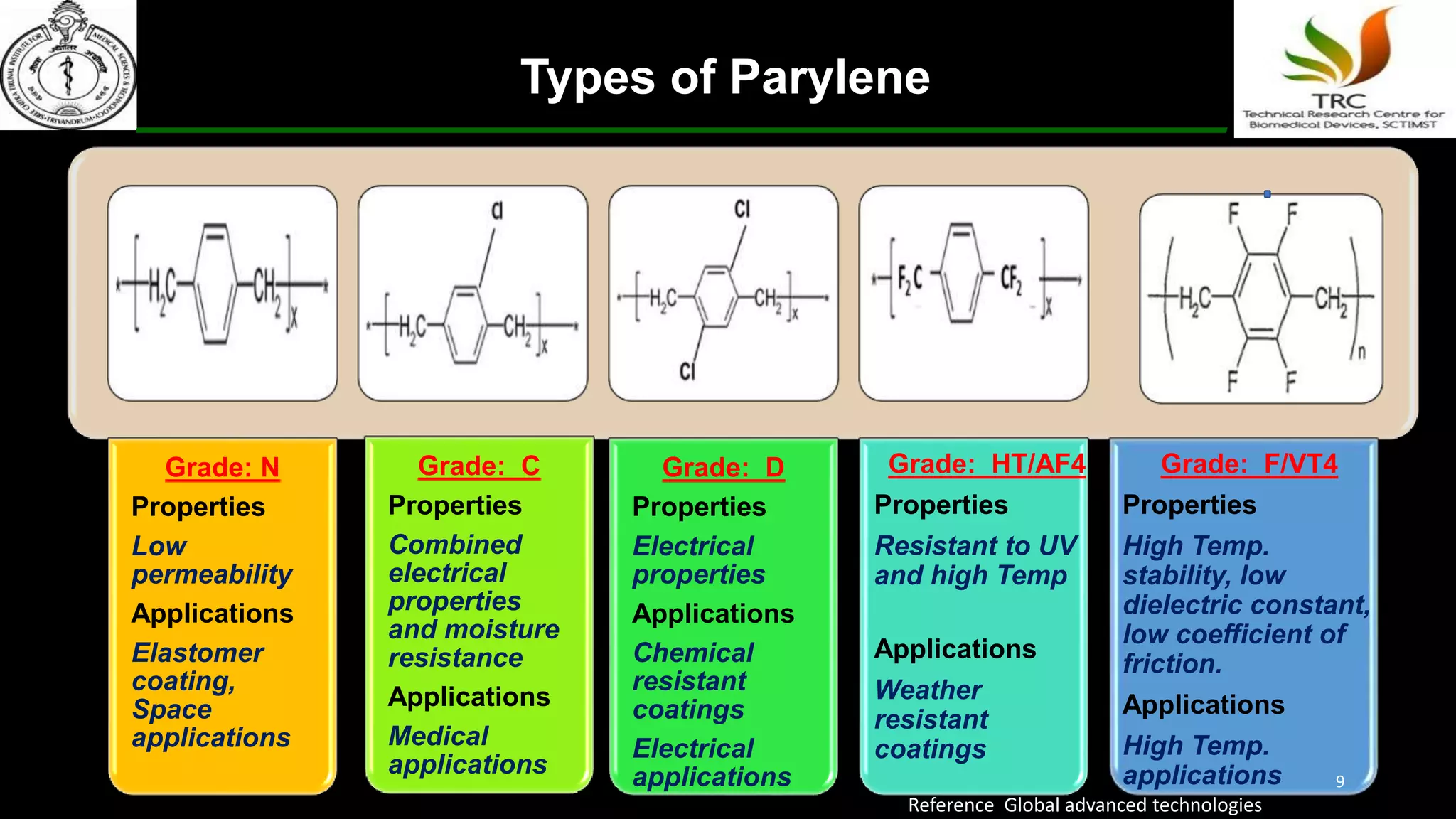
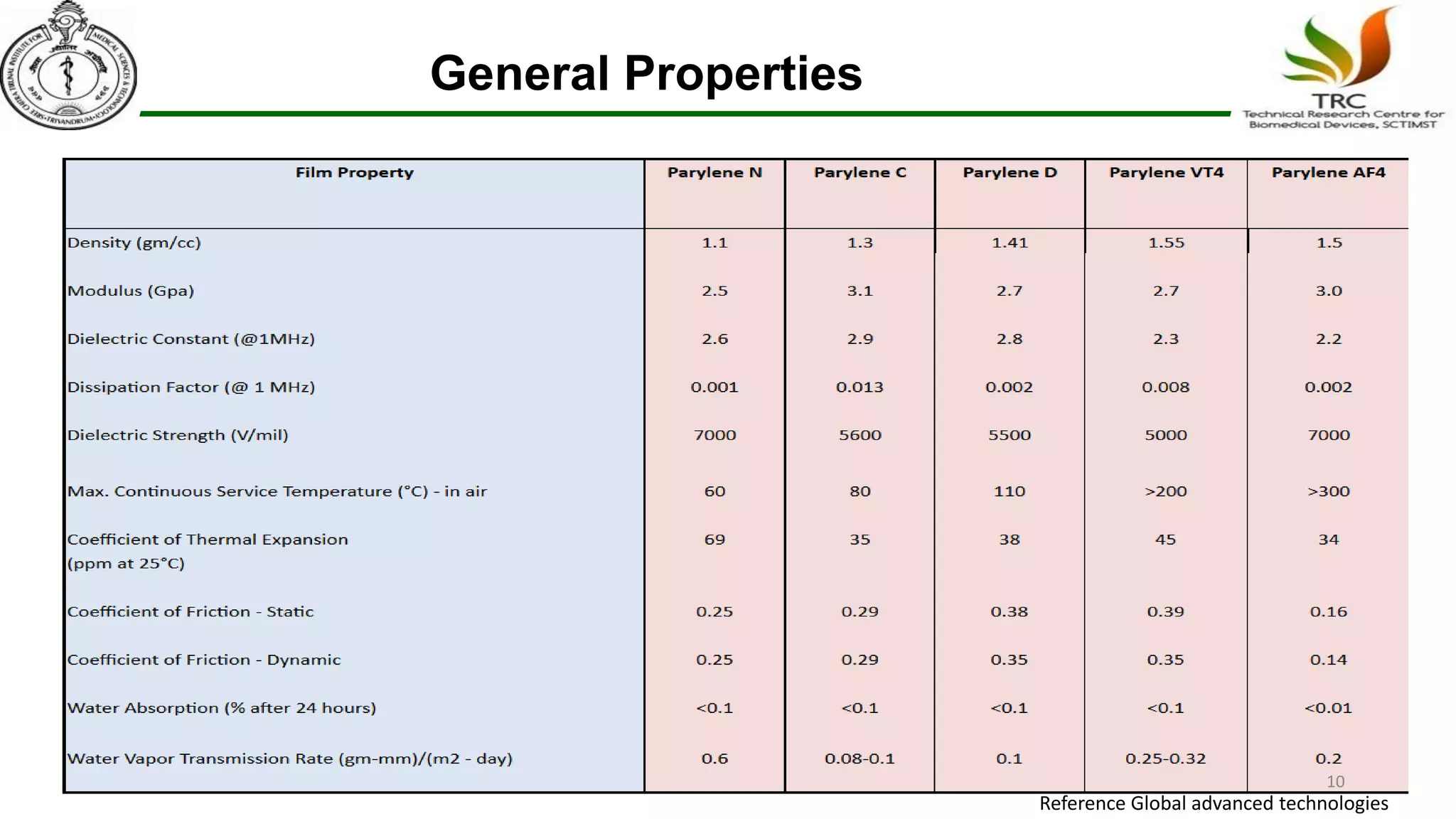
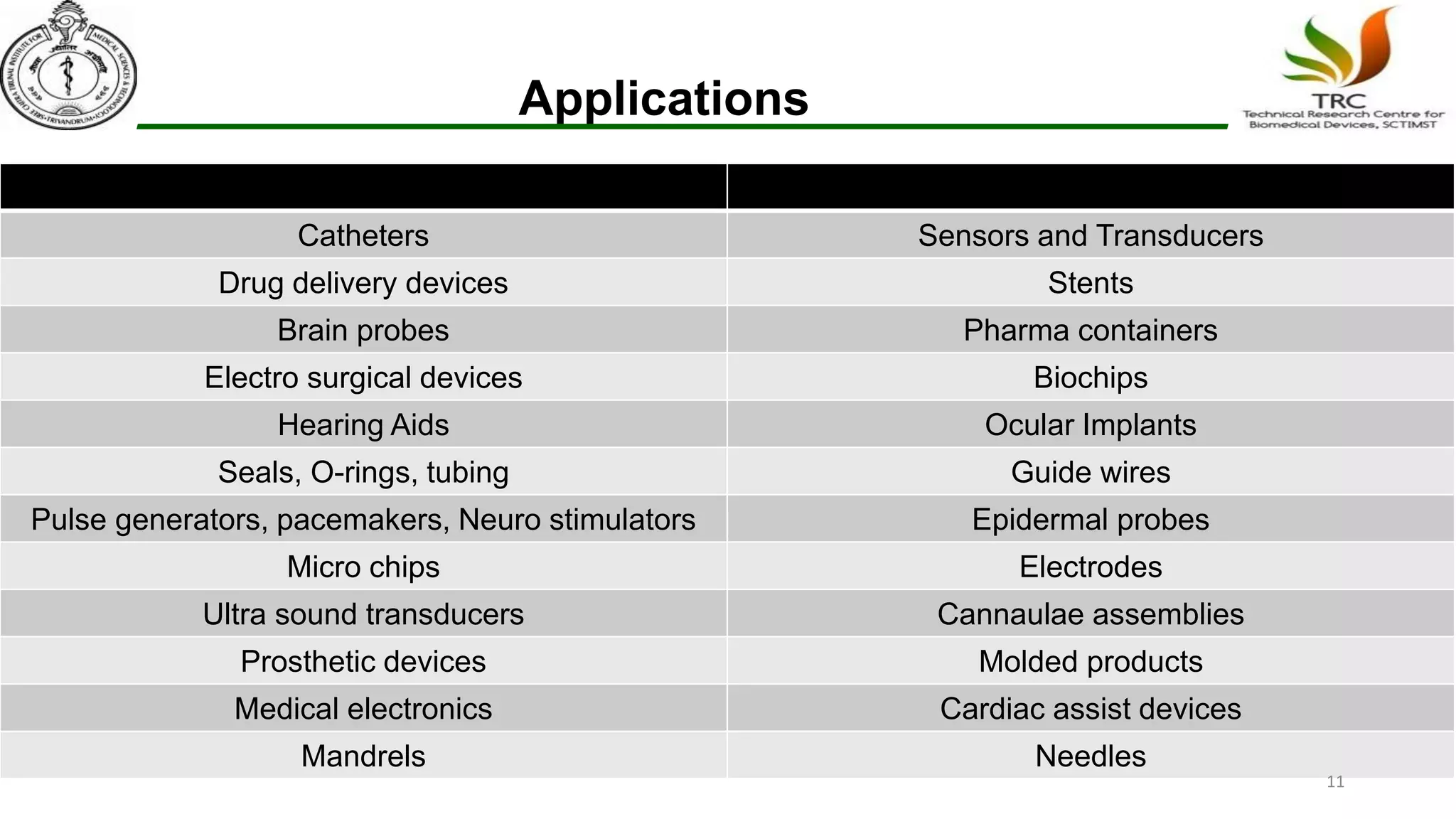
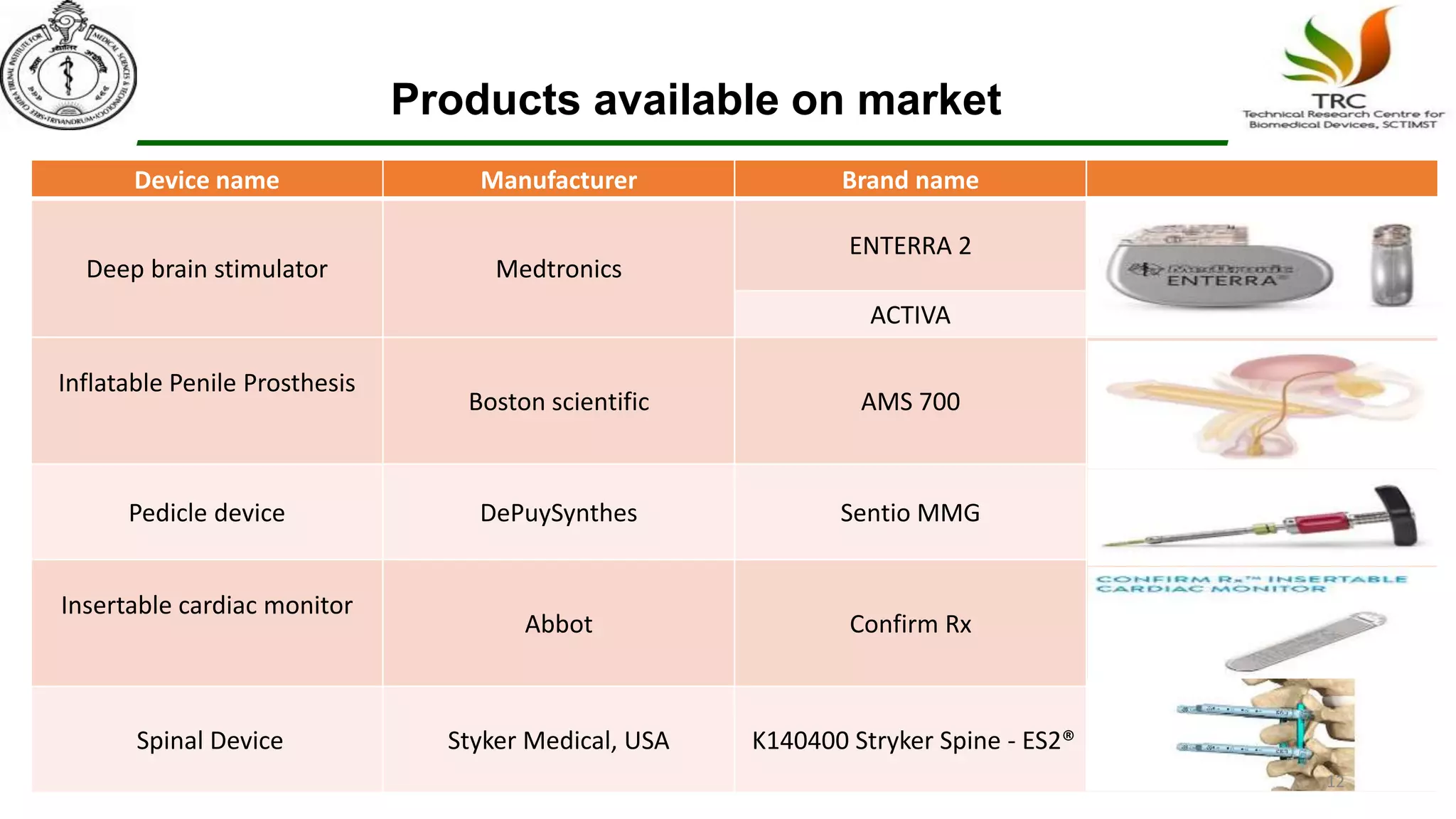
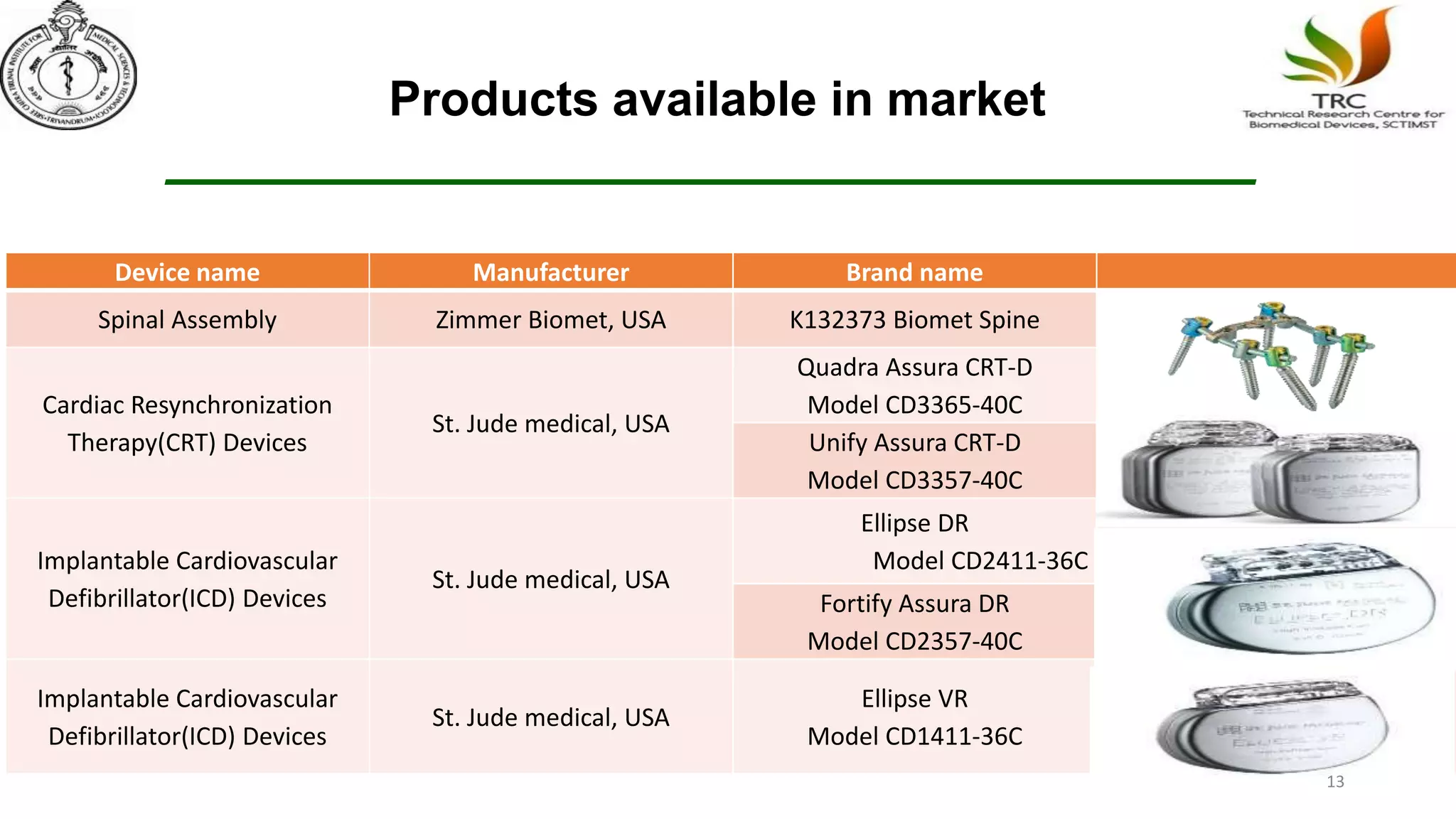
Parylene is a biocompatible coating material well-suited for medical devices due to its conformal coating abilities, chemical resistance, and stability at high temperatures. It forms pinhole-free, uniform films through a vapor deposition process. There are multiple types of Parylene for various applications, and it is used to coat a wide range of implantable medical devices from catheters and stents to neurostimulators, pacemakers, and more. Parylene's bioinertness and ability to withstand sterilization make it a popular choice for enhancing the safety and longevity of medical implants and devices.