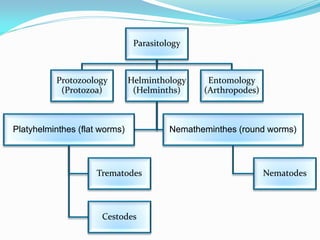
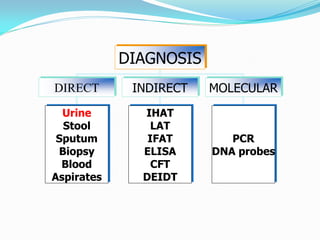
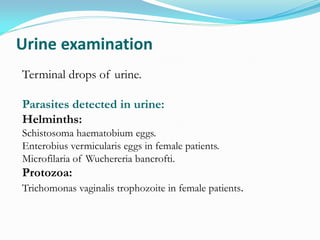
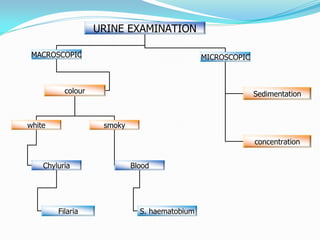
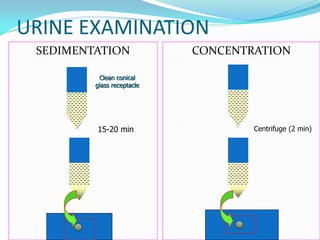
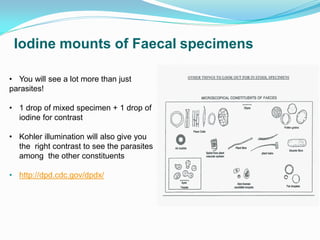
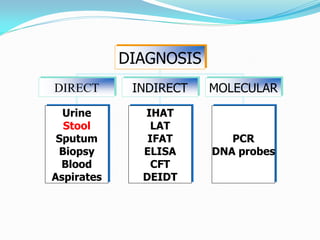
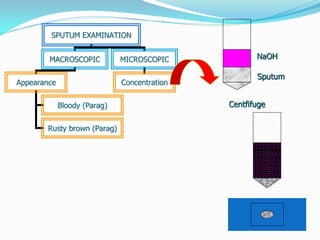
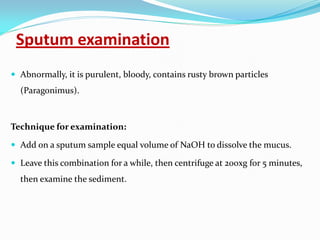
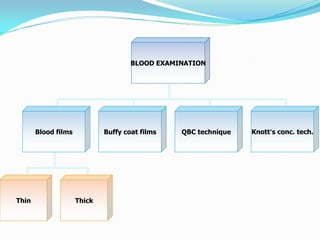
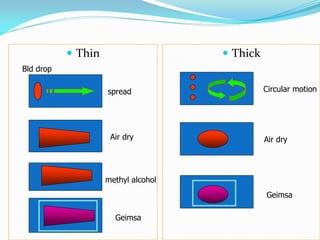
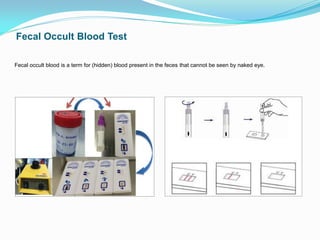
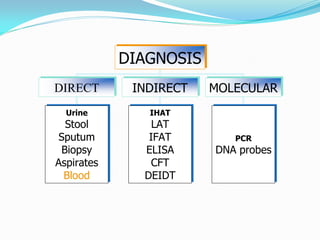
This document provides information on techniques for diagnosing parasites through examination of various specimens. It discusses direct microscopic examination of urine, stool, sputum, and blood films to detect parasites, eggs, or larvae. It also covers concentration techniques used to increase detection rates, such as sedimentation of urine and flotation methods for stool. Indirect methods like serology tests and molecular techniques such as PCR are also mentioned. Precautions for collection, transport, and preservation of specimens are provided to optimize recovery of parasites and morphology.