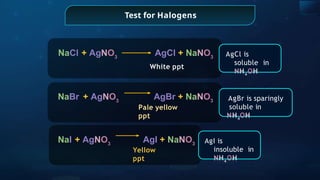
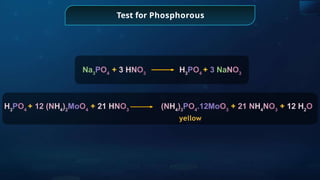
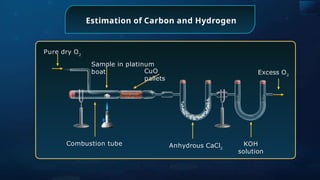
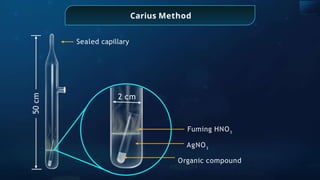
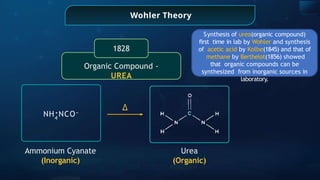
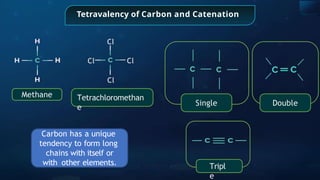
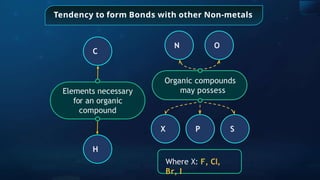
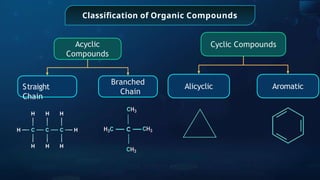
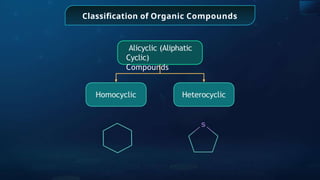
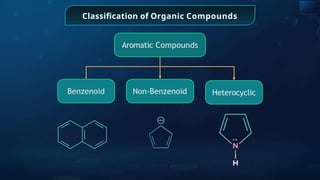
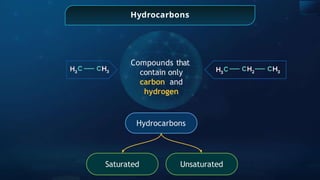
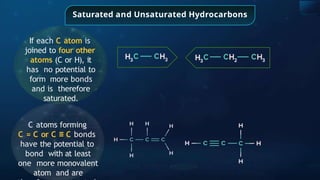
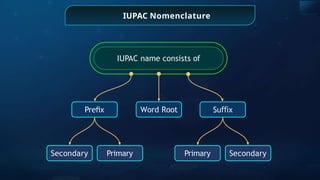
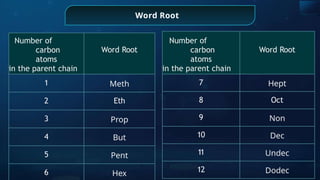
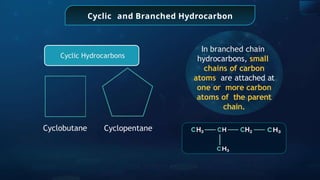
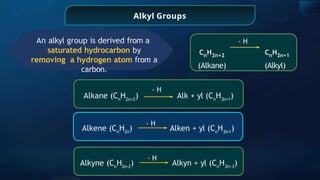
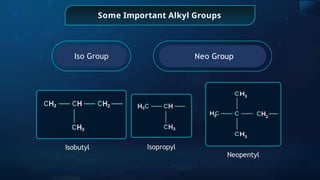
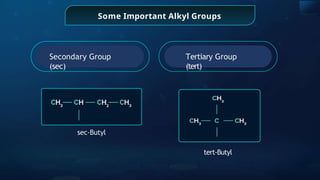
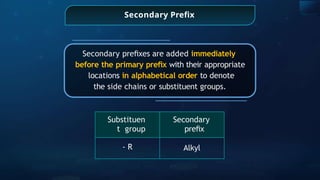
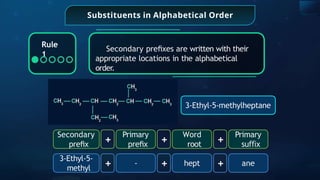
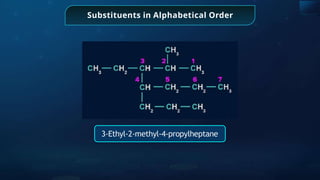
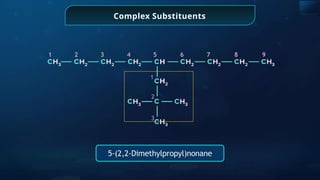
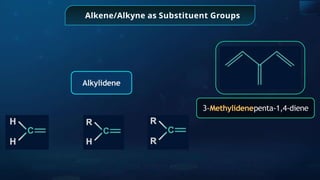
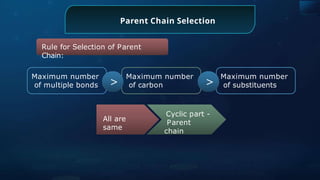
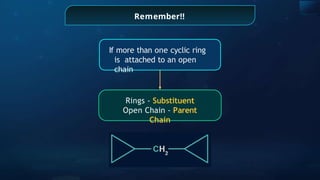
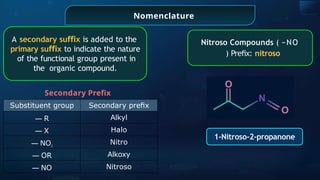
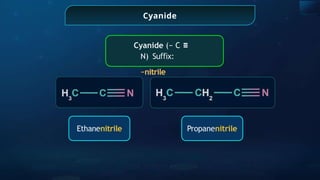
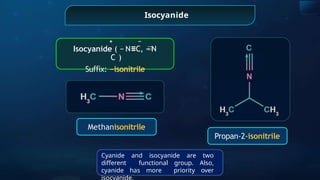
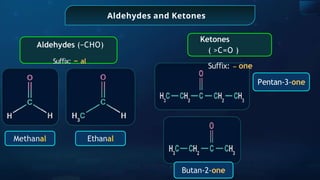
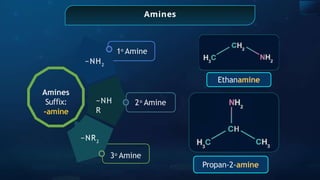
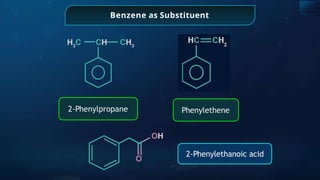
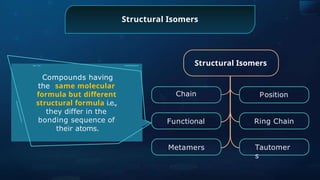
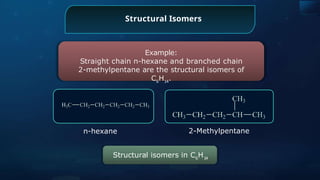
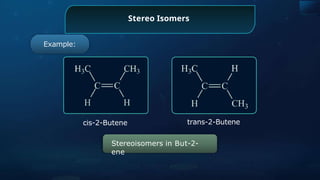
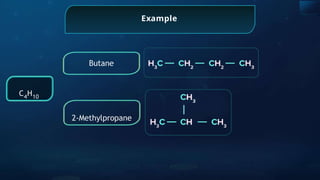
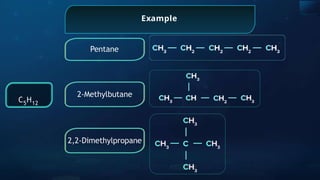
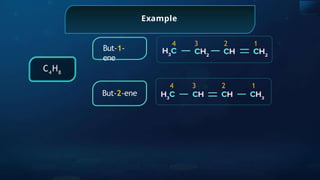
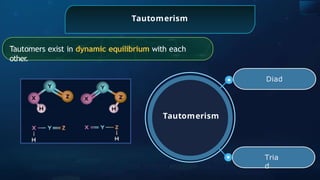
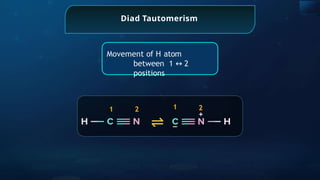
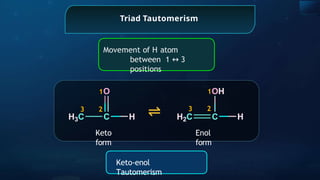
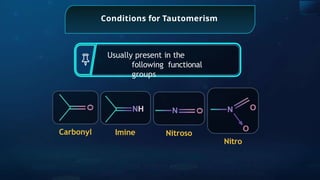
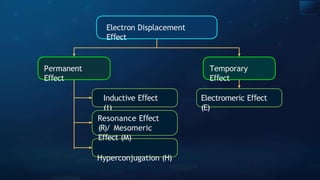
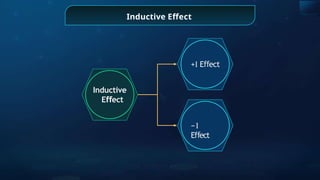
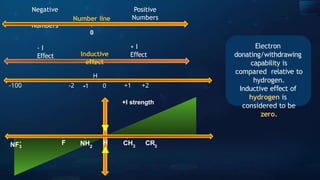
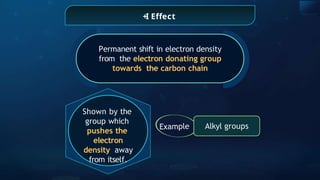
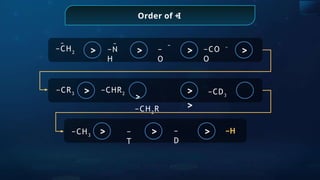
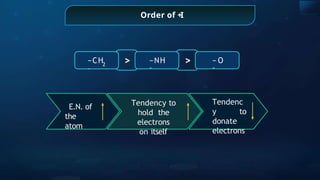
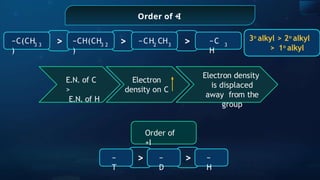
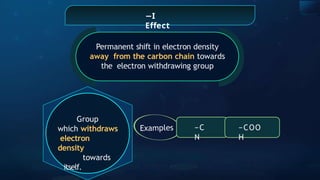
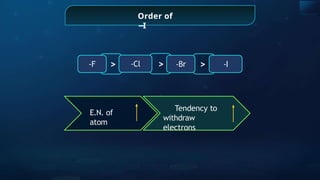
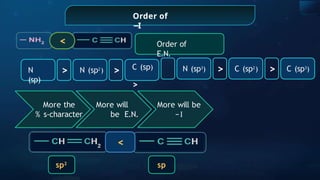
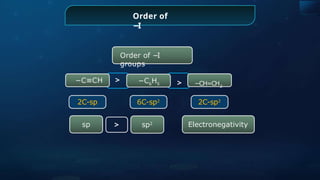
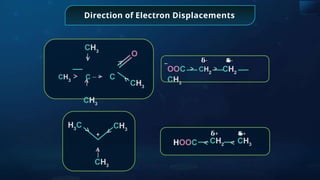
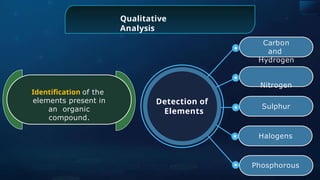
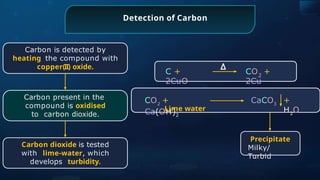
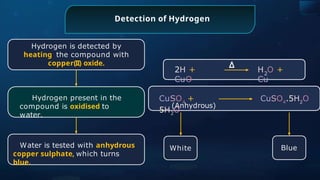
The document provides an extensive overview of organic chemistry, focusing on the classification, nomenclature, and properties of organic compounds, particularly hydrocarbons and their derivatives. It discusses key concepts like functional groups, structural representations, and the IUPAC naming conventions for various types of organic compounds, including alkanes, alkenes, and other functional groups. The document serves as a foundational guide for understanding organic chemistry principles and techniques.
























































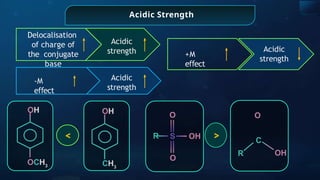
![Acid Dissociation Constant for HA
[HA]
a
K
=
_ [H+
] [A
_
]
+ pKa
-log Ka
=
HA ⇌ H + A
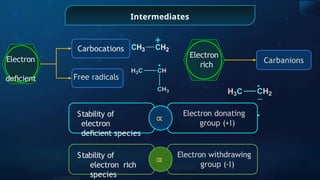
Acidic
strength
Stability of the
conjugate
base
∝
∝
∝
1
Presence of E.D.G.
(+I)
Presence of E.W.G. (-
I)](https://image.slidesharecdn.com/goc-241029172149-a3b0d71f/85/Organic-chemistry-some-basic-principles-and-techniques-GOC-pptx-201-320.jpg)
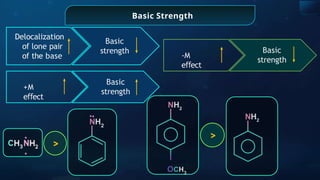
![For a Base ‘B’
2
B + H O ⇌
pKb
– log Kb
Kb
[ B
]
=
=
BH+
+ OH
_
_
[BH+
] [OH
]
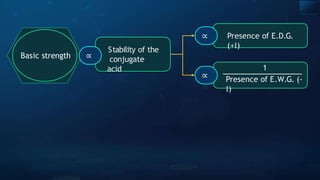
Basic
strength
Stability of the
conjugate
acid
∝
∝
∝
1
Presence of E.W.G. (-
I)
Presence of E.D.G.
(+I)](https://image.slidesharecdn.com/goc-241029172149-a3b0d71f/85/Organic-chemistry-some-basic-principles-and-techniques-GOC-pptx-202-320.jpg)


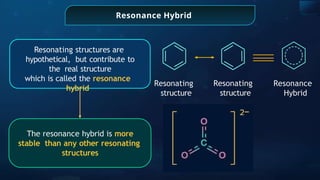

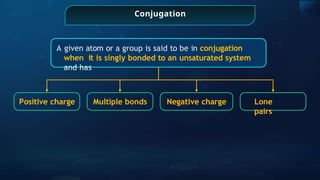
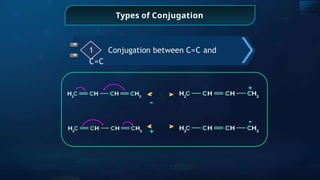
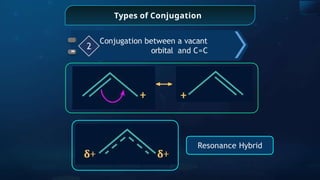


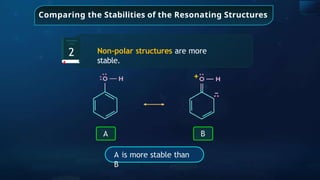
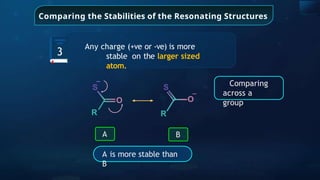
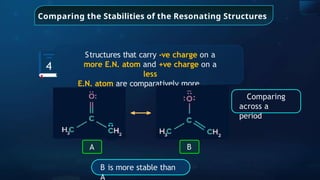
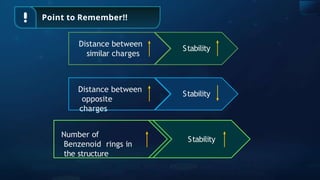
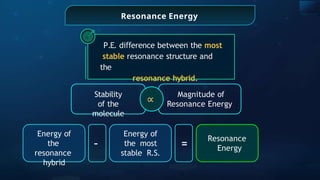

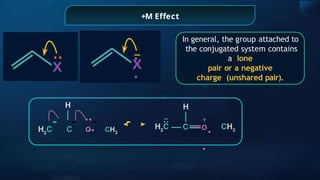
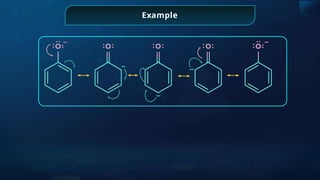

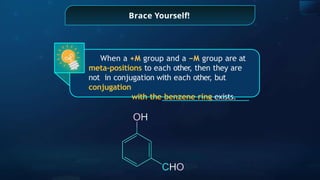

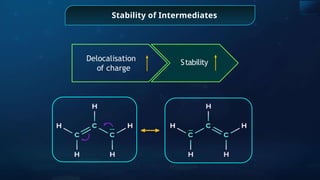
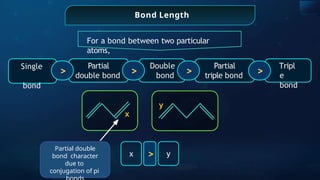
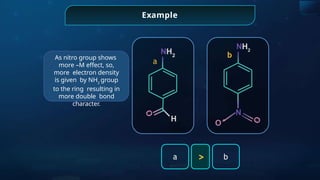



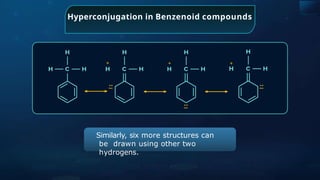
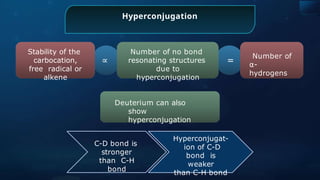



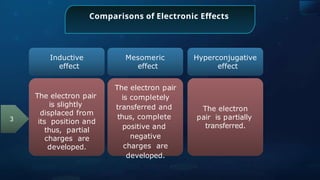
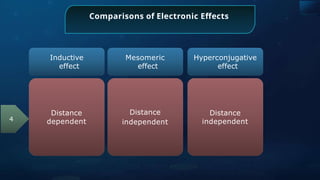


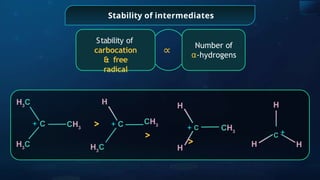
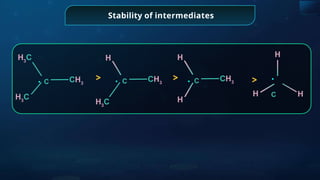



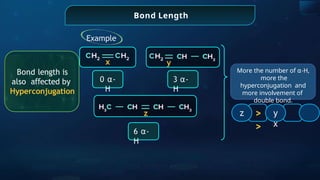
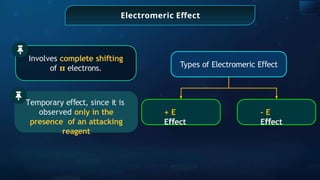
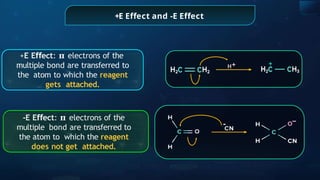
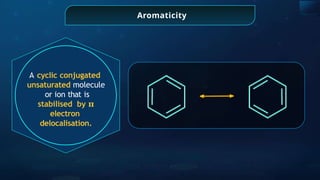


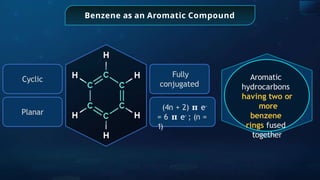


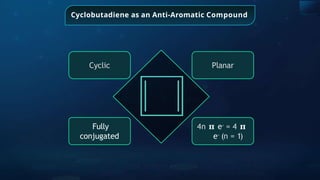
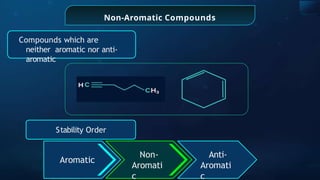
![Annulene
It is the general name of
monocyclic systems
having conjugated
polyenes
[8] -
Annulene
Ring size of annulene is
indicated by number in
bracket
Example:](https://image.slidesharecdn.com/goc-241029172149-a3b0d71f/85/Organic-chemistry-some-basic-principles-and-techniques-GOC-pptx-283-320.jpg)







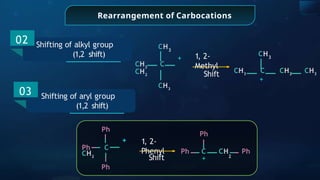
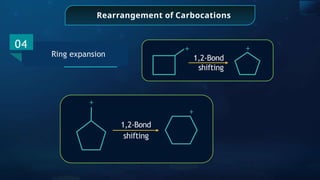




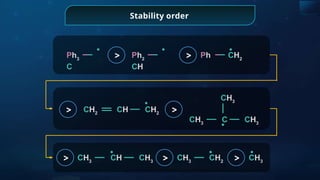



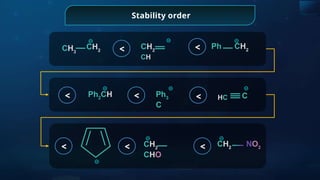






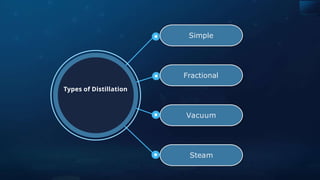

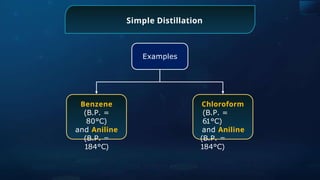

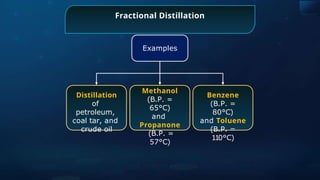









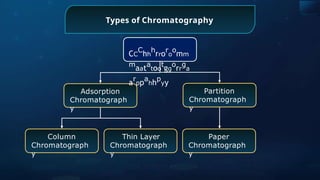





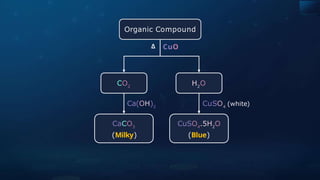

![Detection of Nitrogen
FeSO4
+ 6NaCN Na4
[Fe(CN)6
]+ Na2
SO4
Sodium hexacyanoferrate
(II)
Δ
0
1
Sodium Fusion Extract is boiled with Iron(II)
sulphate
to form Sodium hexacyanoferrate (II).
02
On heating with sulphuric acid, some
Fe2+
ions are oxidised to Fe3+
ions.](https://image.slidesharecdn.com/goc-241029172149-a3b0d71f/85/Organic-chemistry-some-basic-principles-and-techniques-GOC-pptx-353-320.jpg)
![Detection of Nitrogen
6 4 6 3 2
Fe [Fe(CN) ] .xH O
3[Fe(CN )]
4-
+ 4Fe3+
xH2
O
Prussian blue
03
Fe3
reacts with Sodium hexacyanoferrate (II) to
give Prussian blue colour of Ferric ferrocyanide,
which confirms the presence of nitrogen.](https://image.slidesharecdn.com/goc-241029172149-a3b0d71f/85/Organic-chemistry-some-basic-principles-and-techniques-GOC-pptx-354-320.jpg)

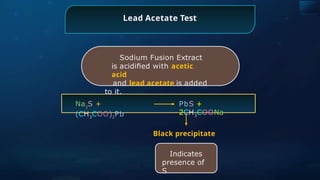
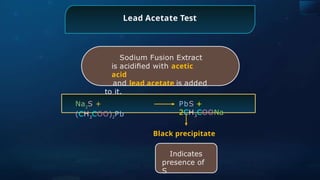
![Sodium Nitroprusside Test
Sodium Fusion Extract is treated
with sodium nitroprusside.
Na2
S +
Na2
[Fe(CN)5
NO]
Na4
[Fe(CN)5
NOS]
Violet
Indicates
presence of
S](https://image.slidesharecdn.com/goc-241029172149-a3b0d71f/85/Organic-chemistry-some-basic-principles-and-techniques-GOC-pptx-358-320.jpg)