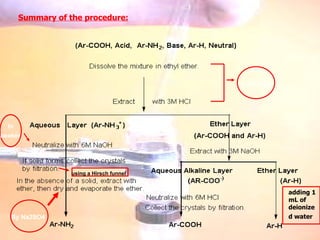
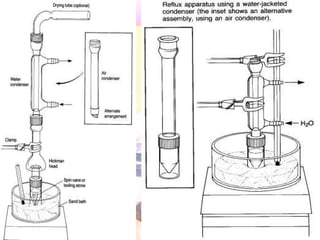
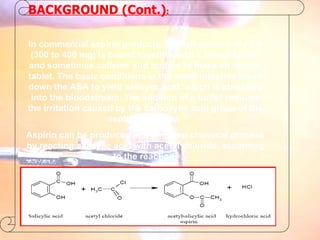
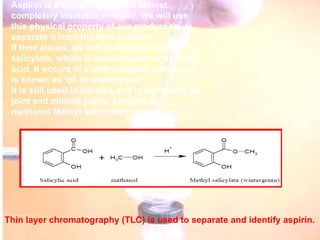
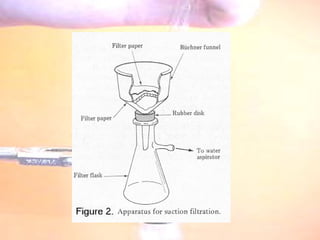
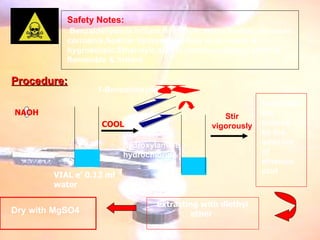
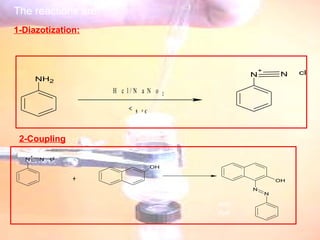
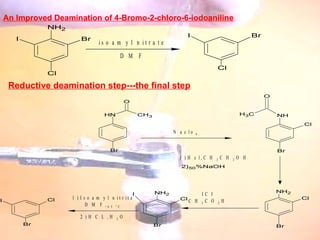
The document provides procedures for microscale synthesis of several organic compounds including aspirin, E-benzal, and azodyes. Microscale chemistry uses small quantities of chemicals to reduce waste and improve safety. It describes basic microscale equipment like conical vials, air condensers, Craig tubes for recrystallization, and pipettes. The aspirin synthesis involves reacting salicylic acid with acetyl chloride to form aspirin, which is then recrystallized. The E-benzal synthesis reacts benzaldehyde with hydroxylamine hydrochloride in the presence of sodium hydroxide.




























![Procedure (cont’d): 5-Combine the organic layer, dry the organic layer with Na2SO4 [i] [1] and evaporate solvent on the steam bath.3 6-The yellow ether solutions remaining in the vials are recombined into the 5 mL vial. Add 1 mL of pure water, shake its content and then allow the layers to settle. Remove and discard the bottom aqueous layer. 7-Add 1 mL of 3 M NaOH into the 5 mL conical vial that has the yellow ether solution (benzoic acid and 9-fluorenone). 8-Cap and thoroughly shake the vial to allow completion of the reaction and the dissolution of the compounds in their respective solvents. Allow the layers to separate. 9-Carefully transfer the bottom layer to a 3 mL conical vial using a Pasteur pipet.To the 3 mL vial add one ml of diethyl ether.Cap and shake its contents.As before, transfer the lower layer, this time to a 10 mL beaker.By now the contents of the beaker should be very pale or colorless.](https://image.slidesharecdn.com/organicpresentationonline-100310154507-phpapp02/85/Organic-41-320.jpg)