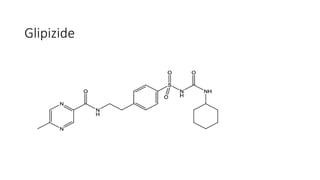
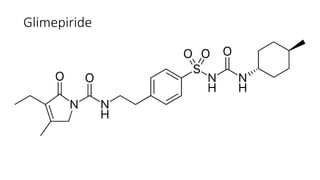
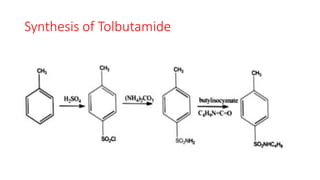
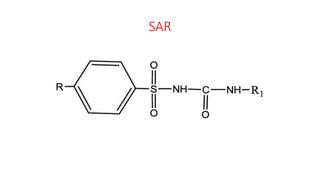
This document classifies and describes various oral hypoglycemic agents used to treat diabetes. It discusses sulfonylureas in depth, including their mechanism of action of stimulating insulin secretion from pancreatic beta cells, and generations of sulfonylureas including chlorpropamide, tolbutamide, glipizide, and glimepiride. It also covers structure-activity relationships for sulfonylureas, noting substituents that enhance antihyperglycemic efficacy.