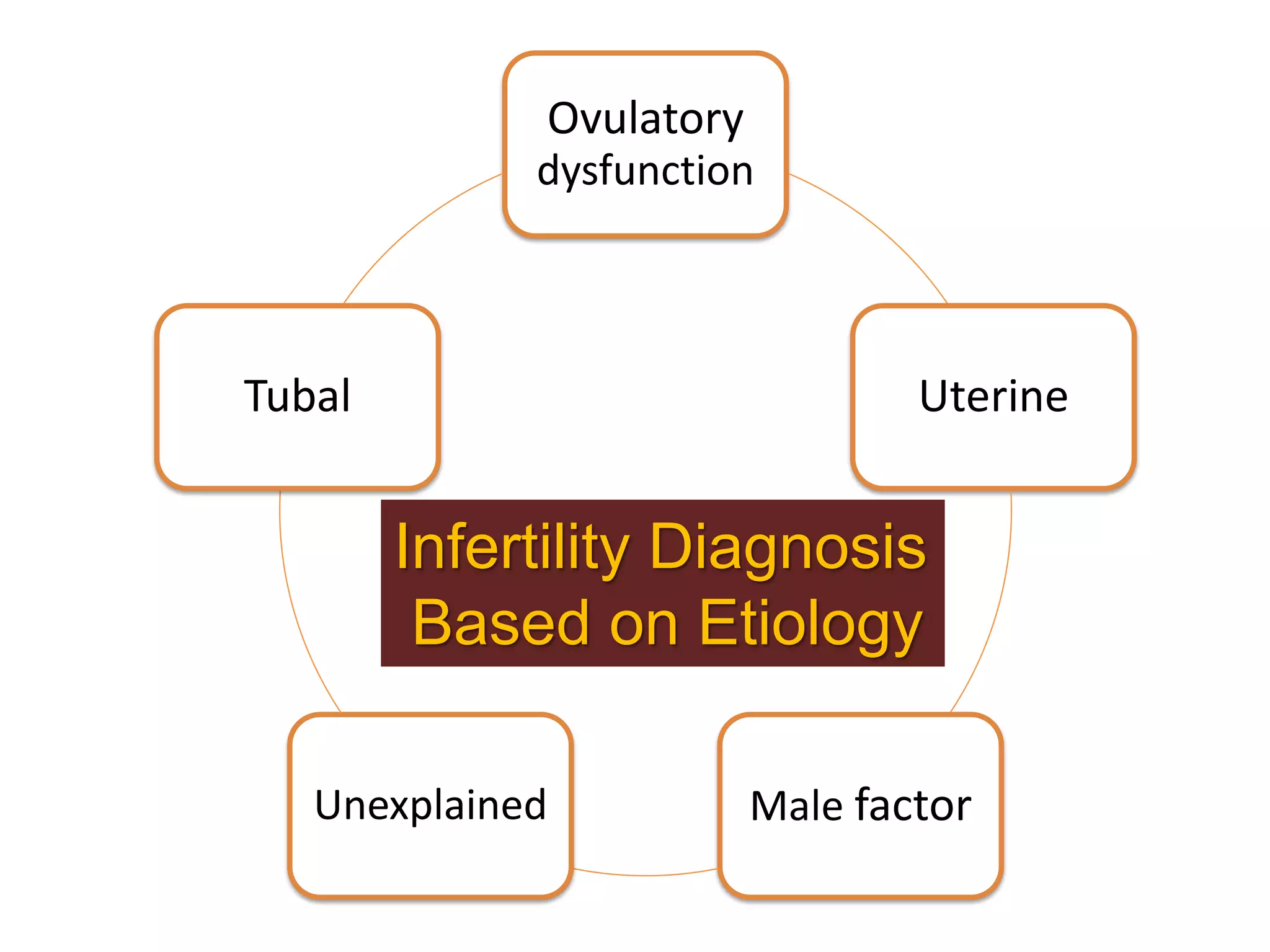
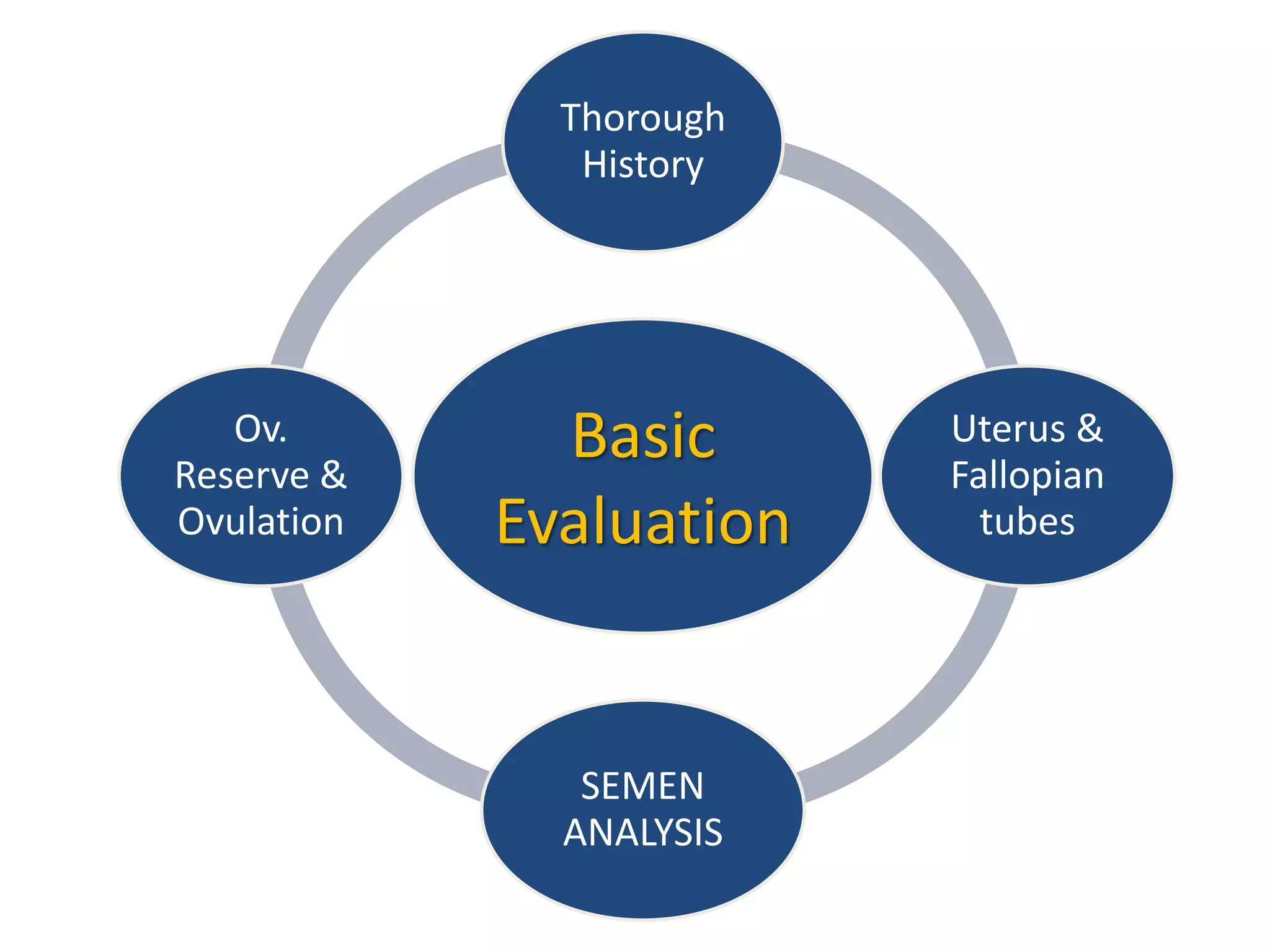
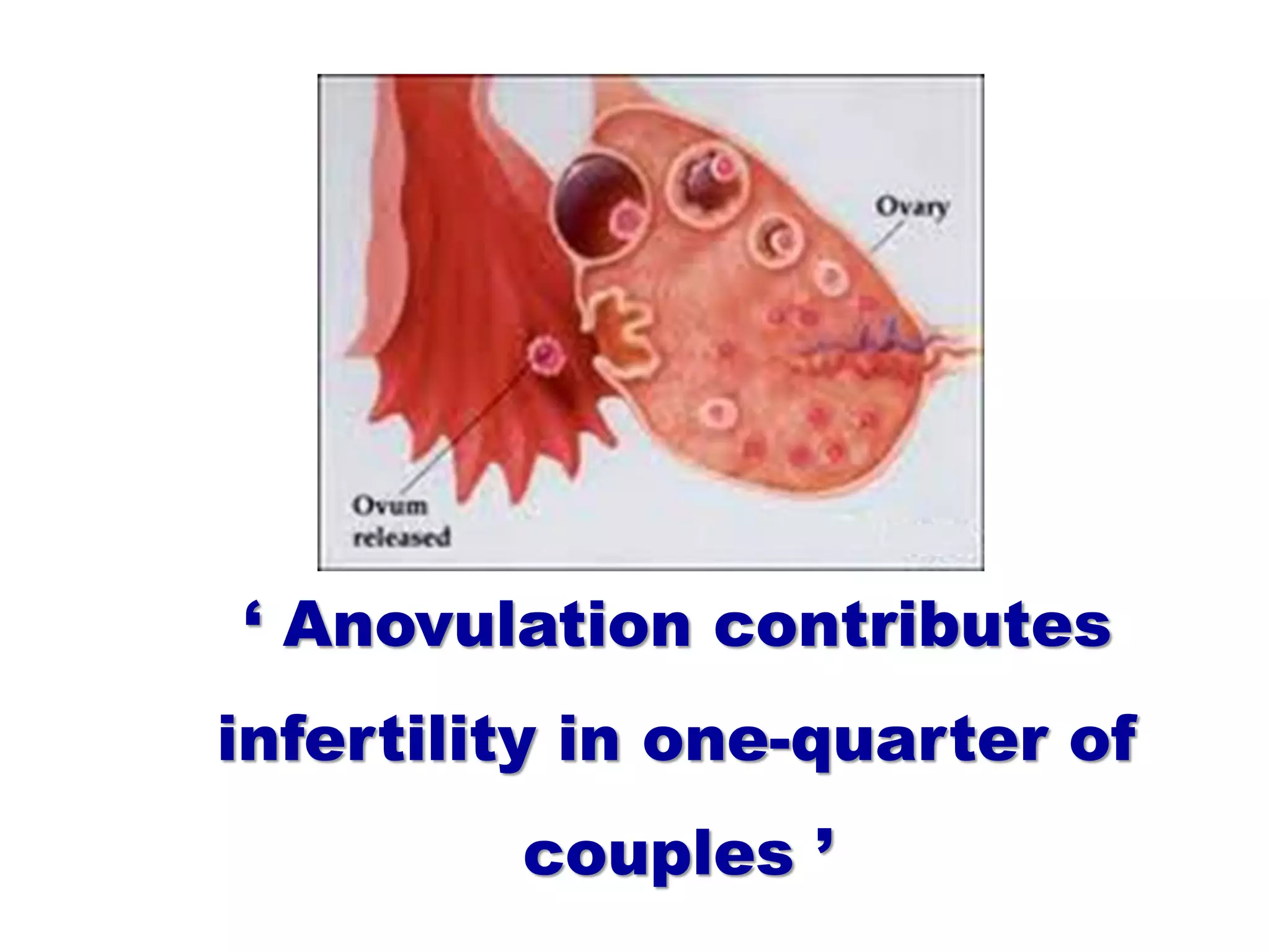
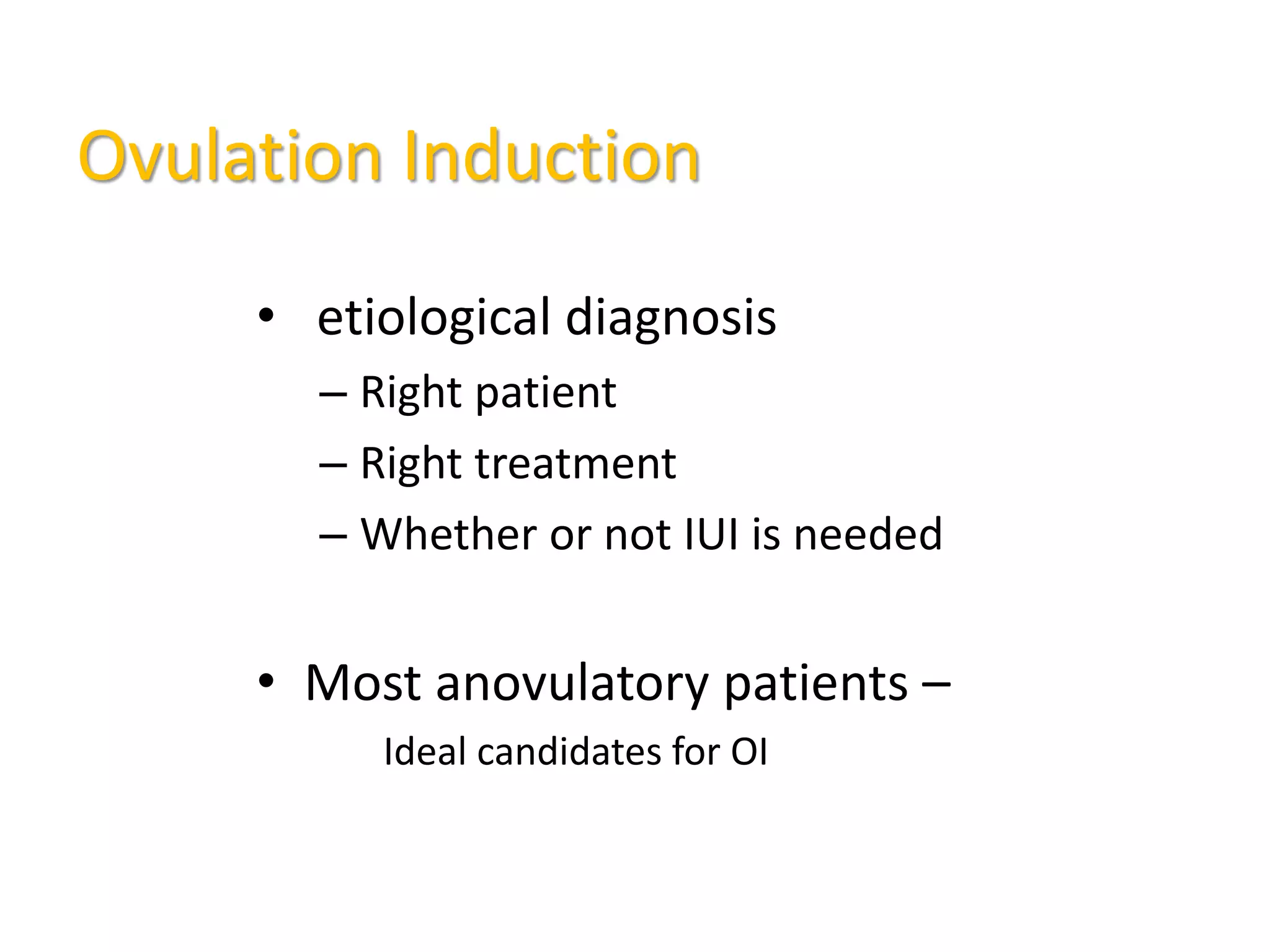
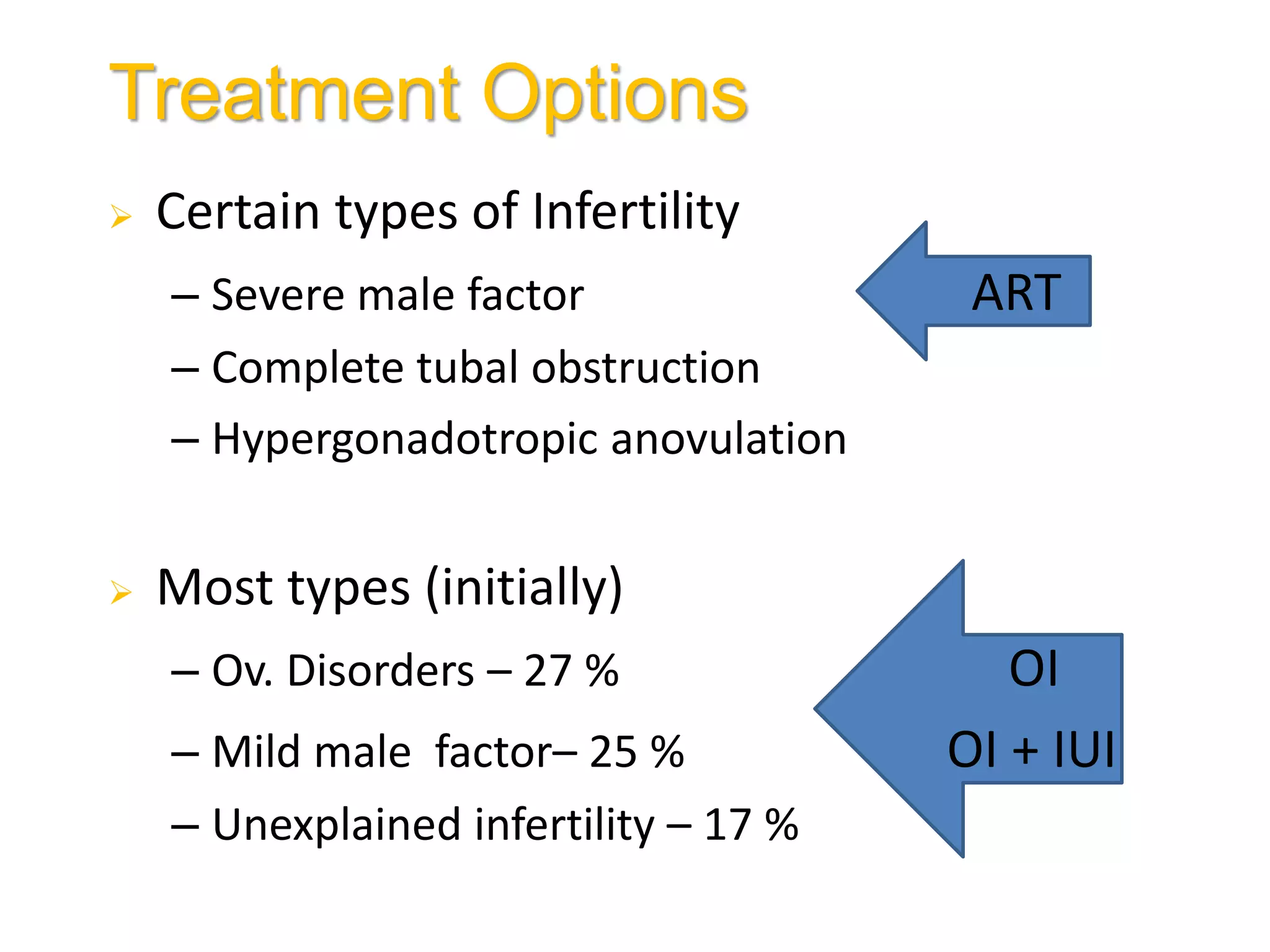
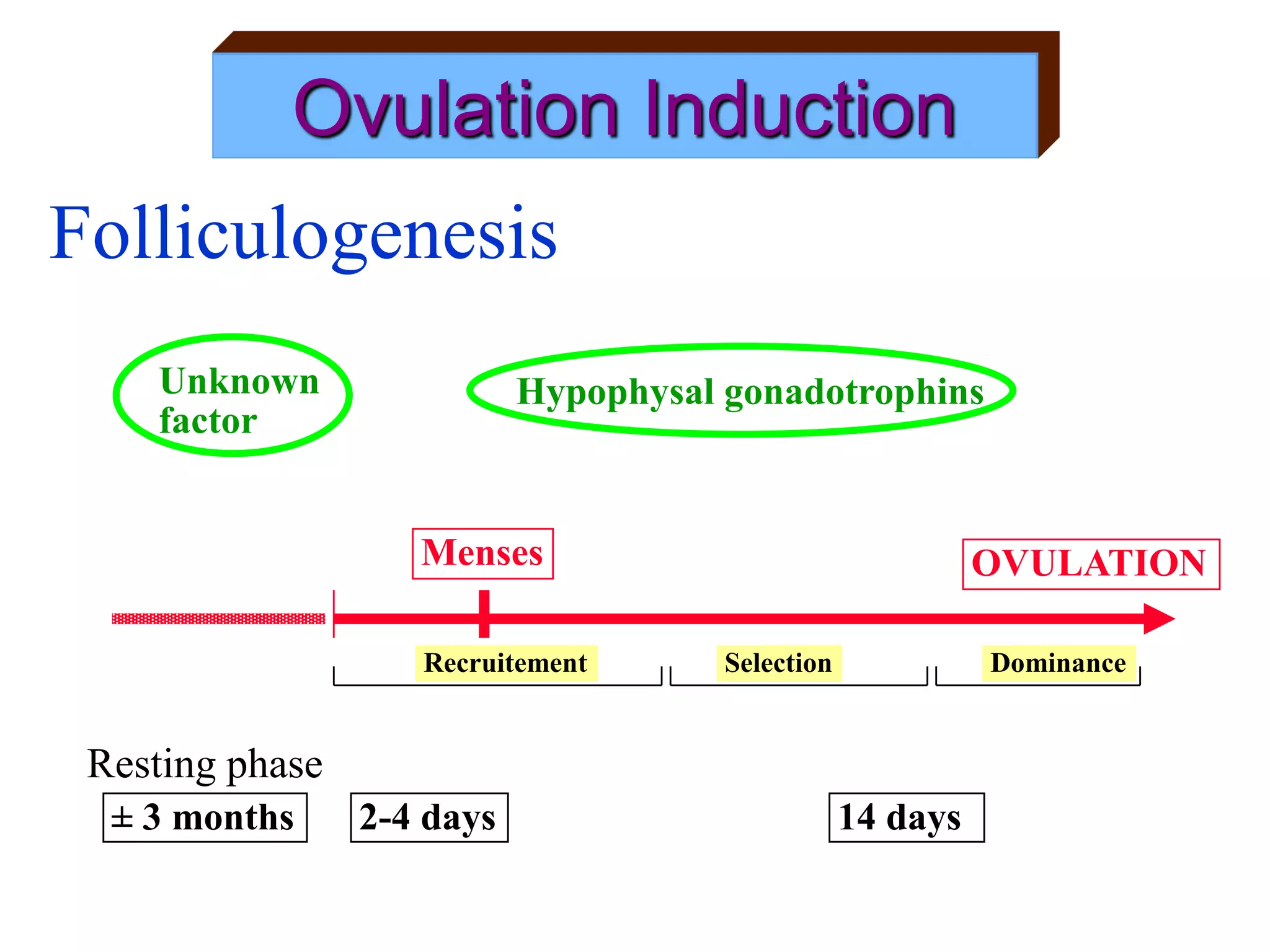
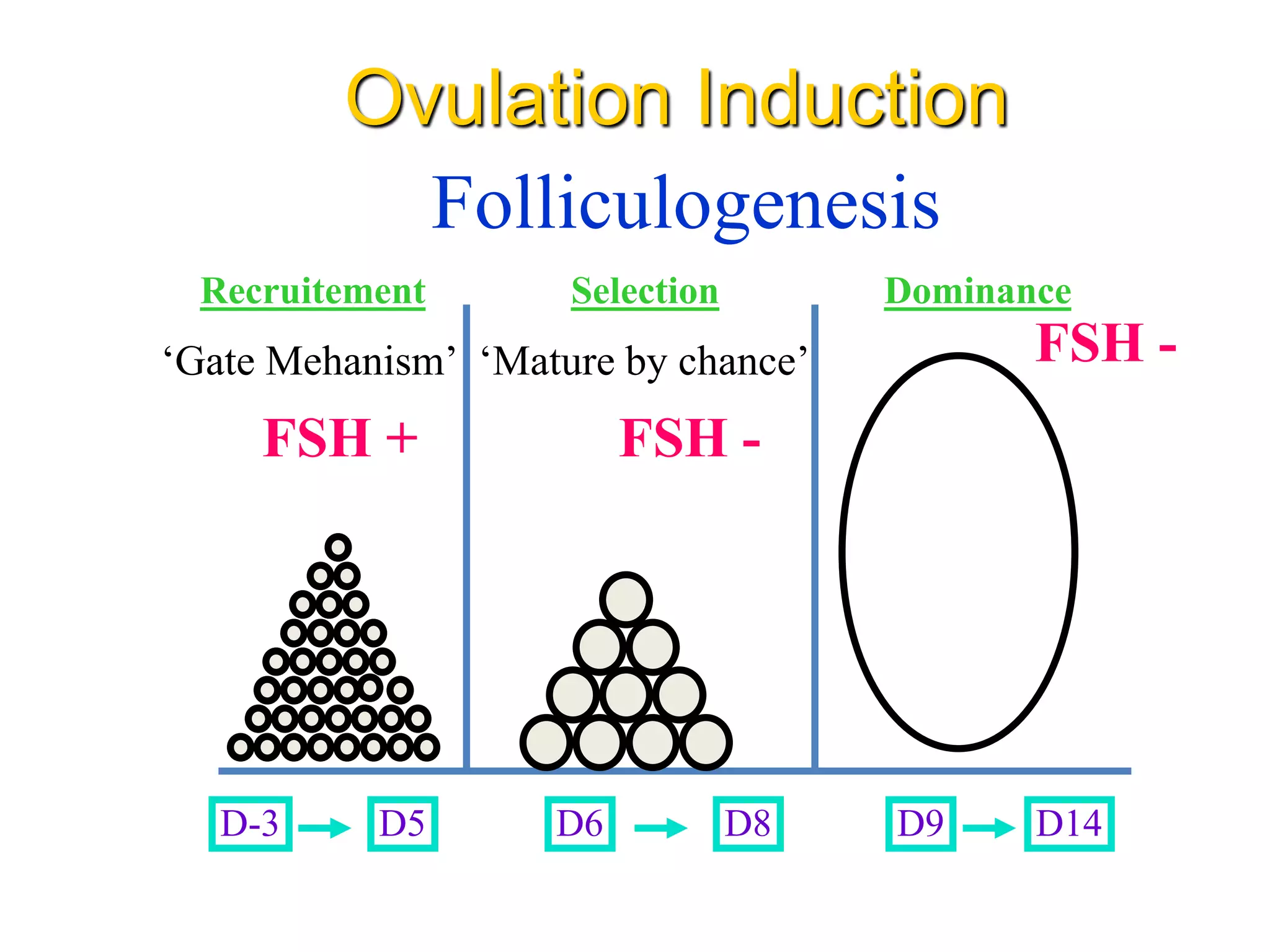
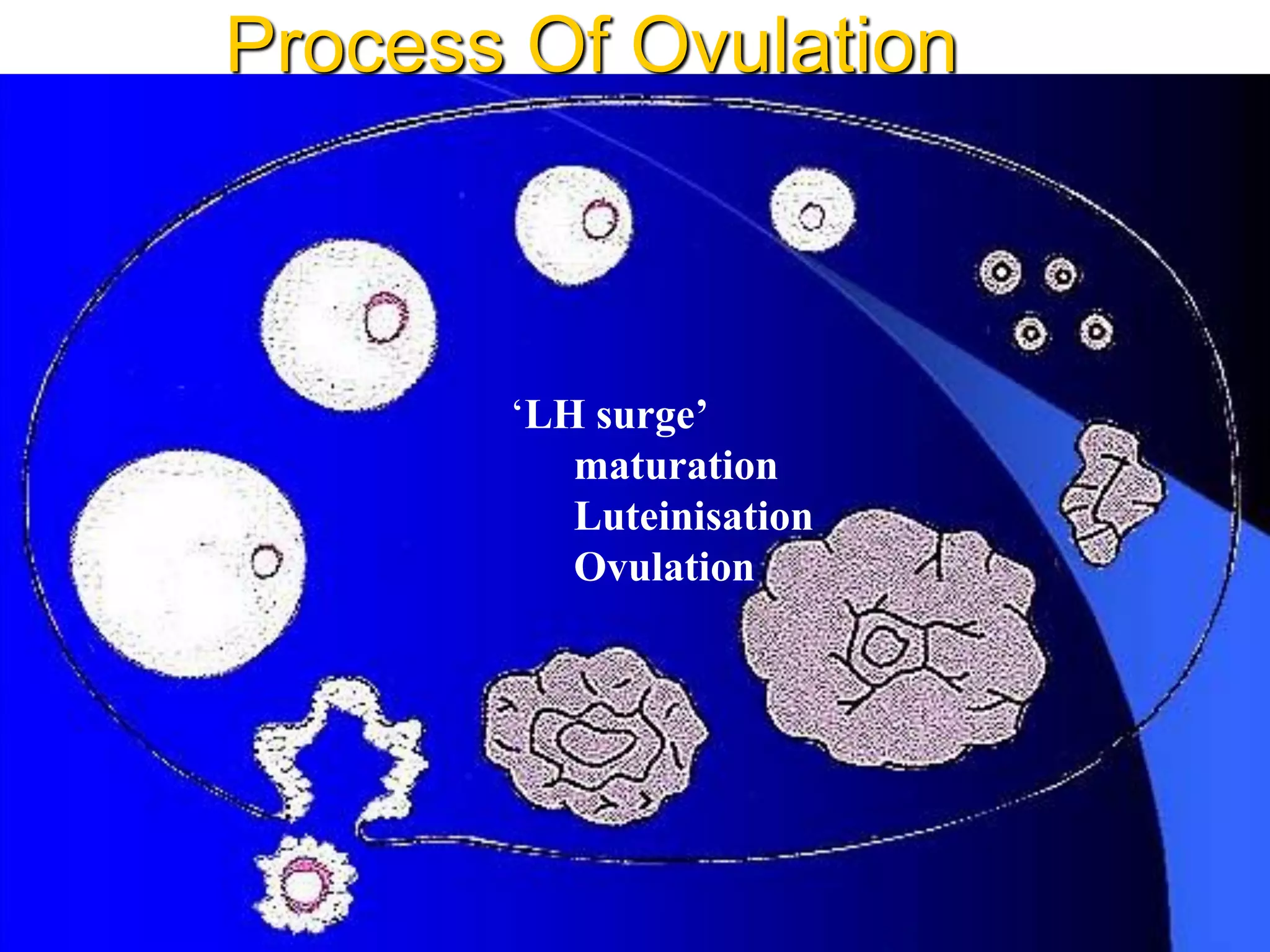
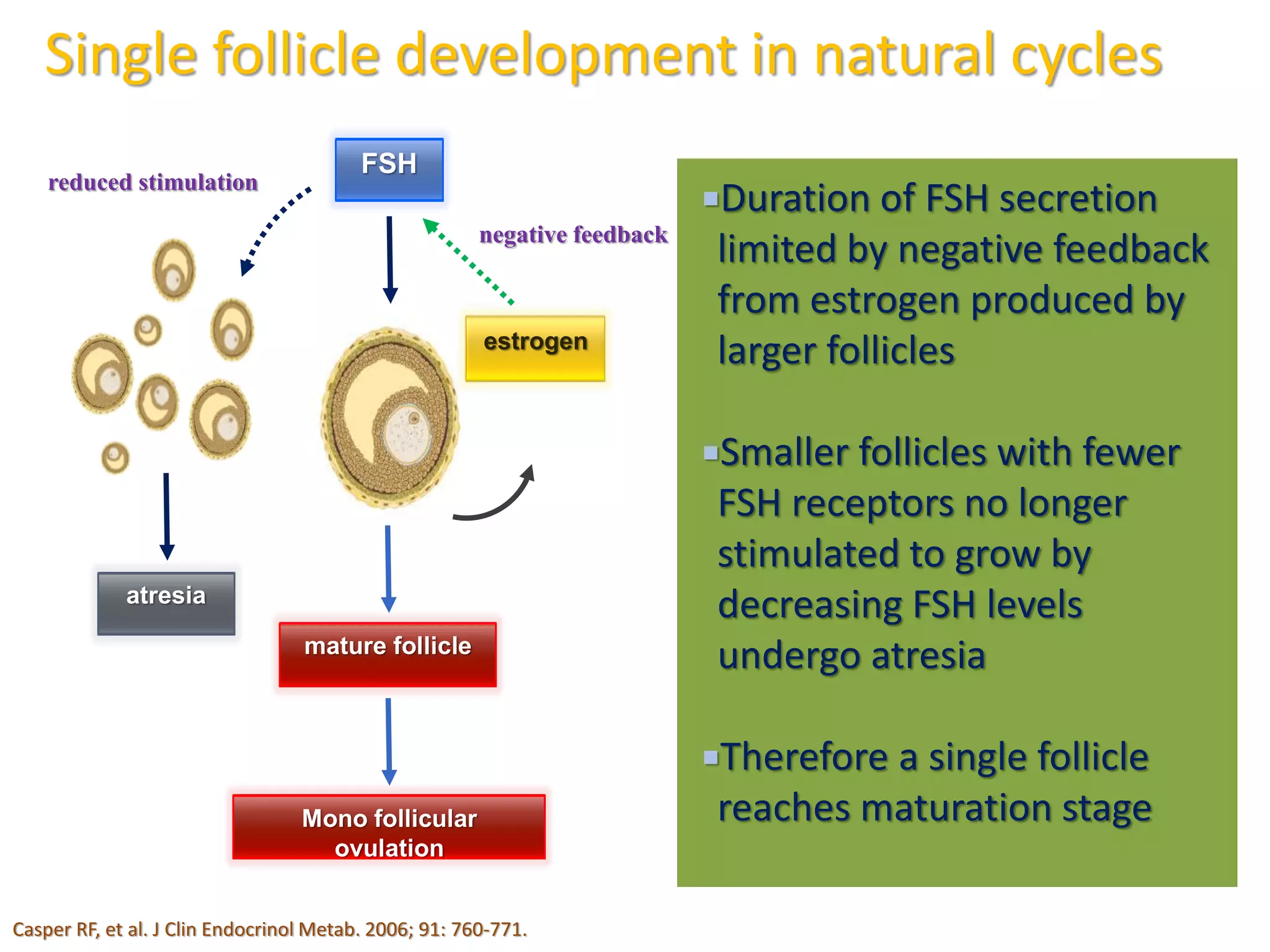
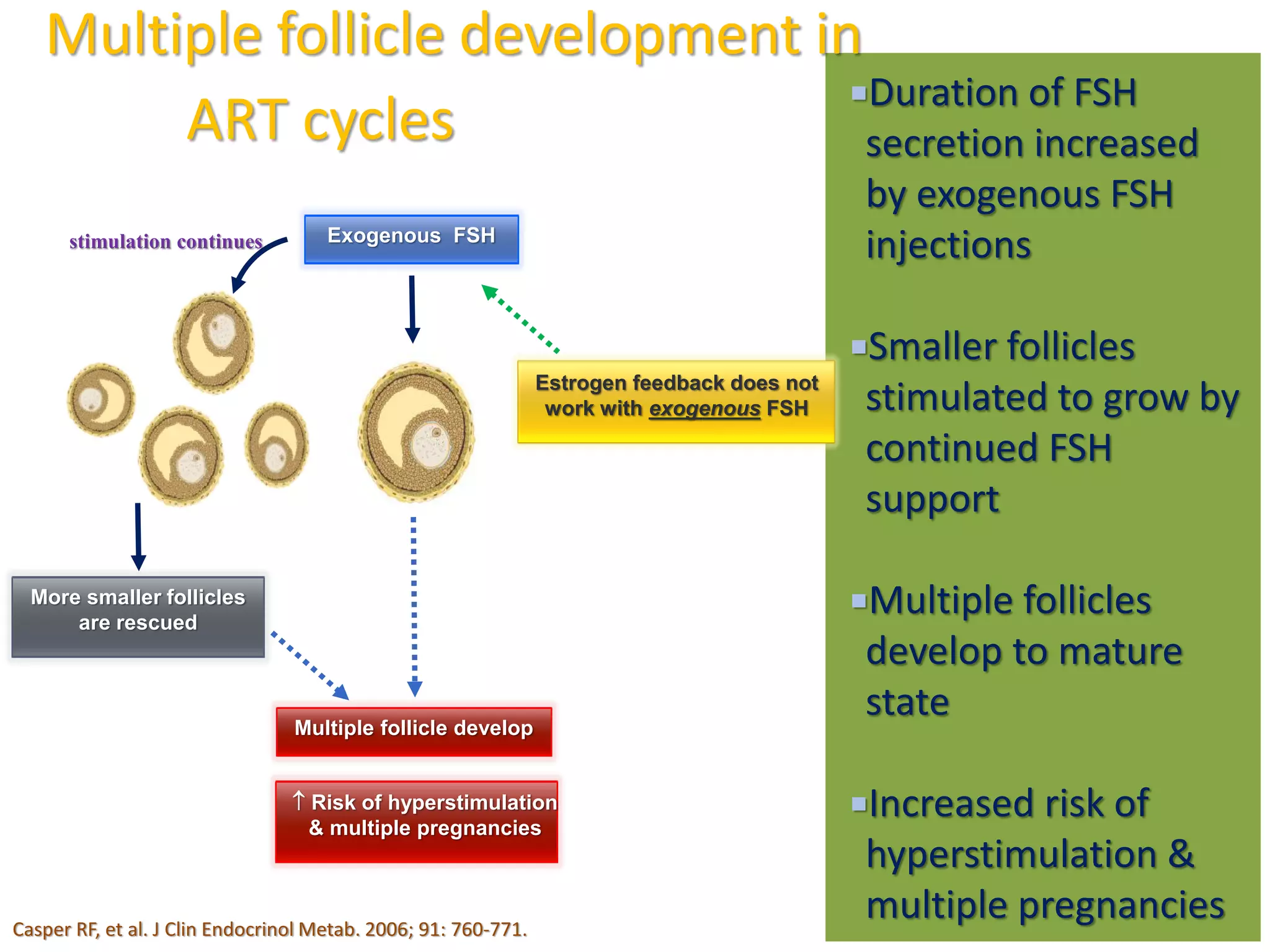
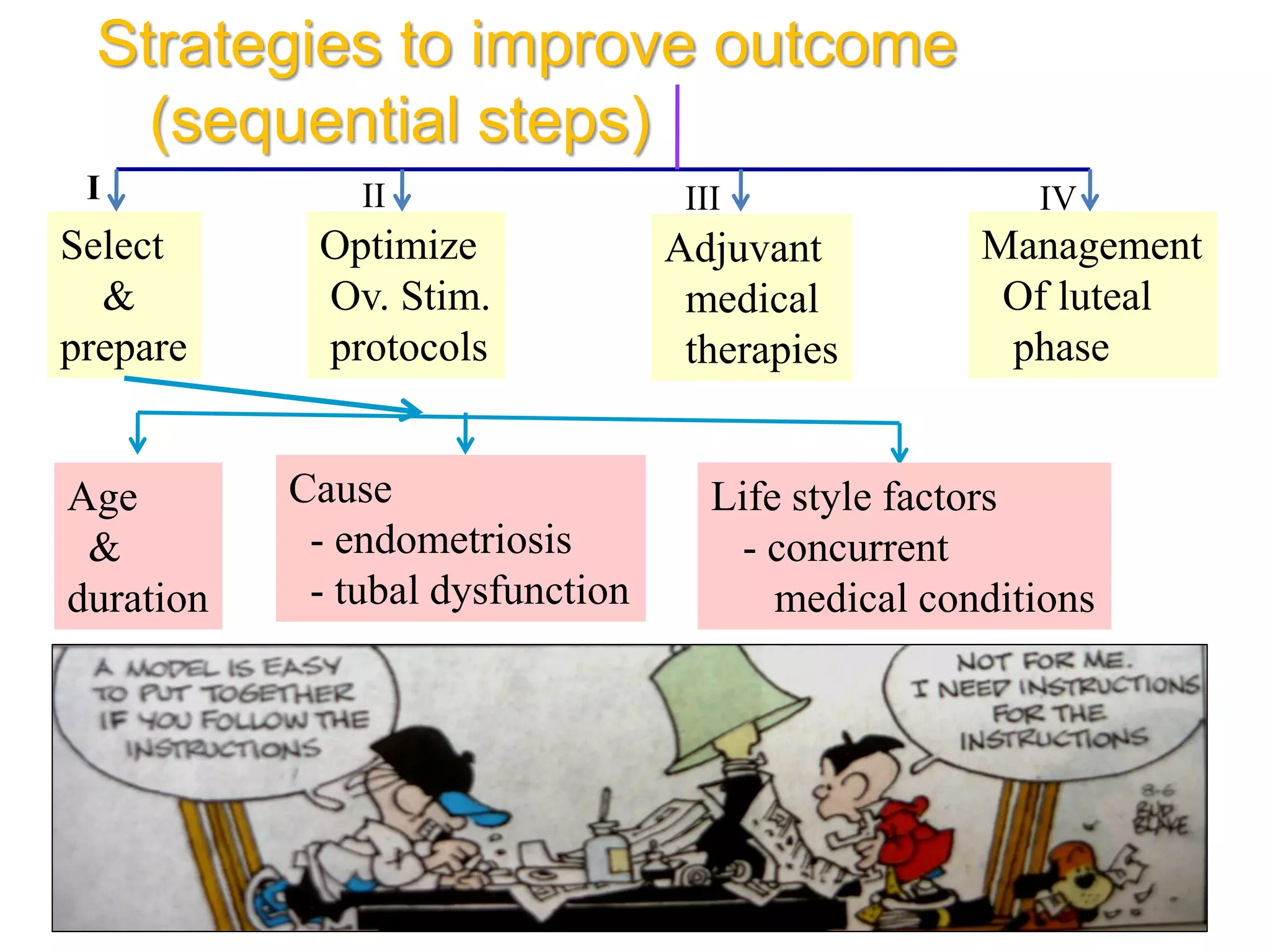
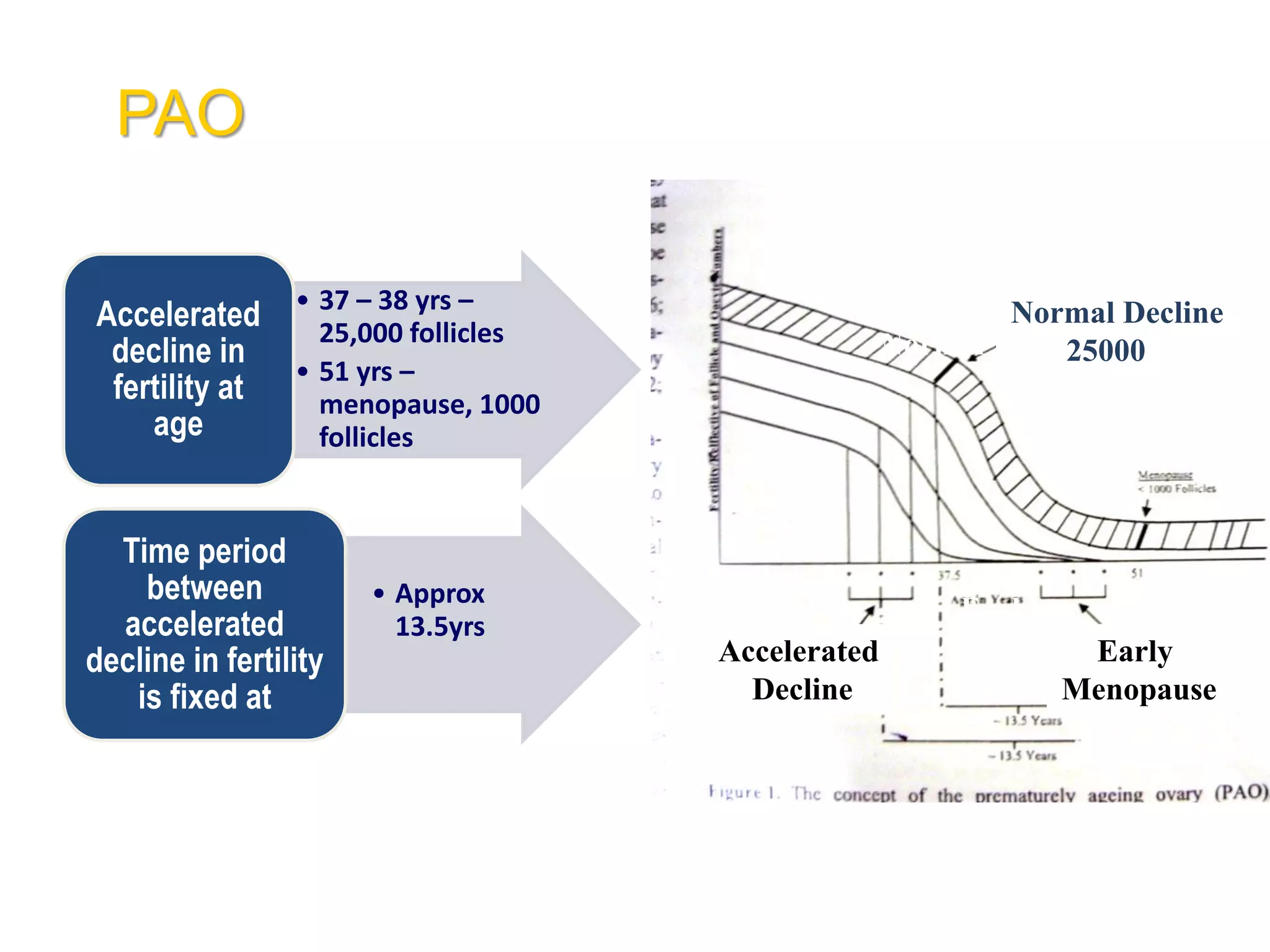
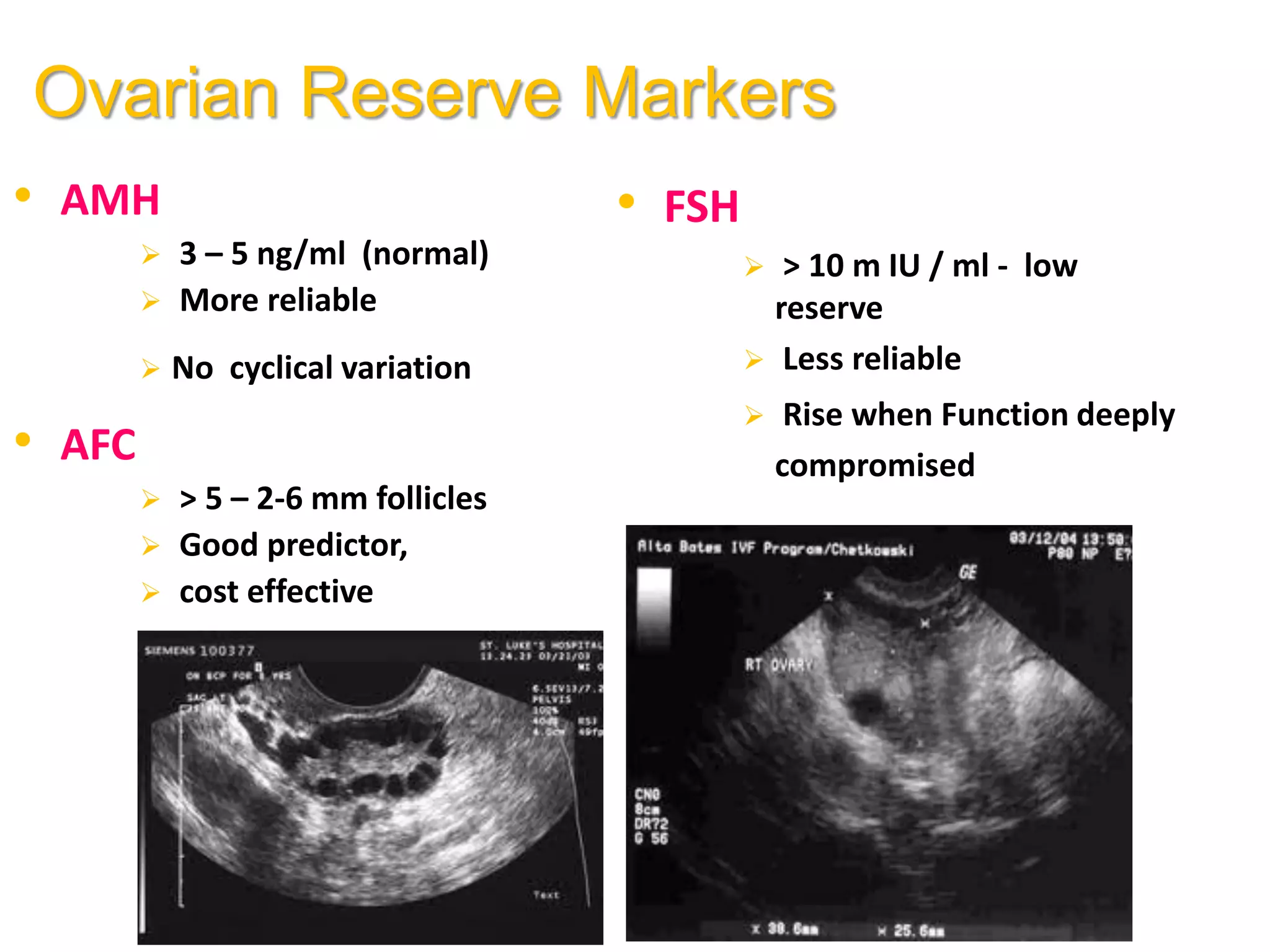
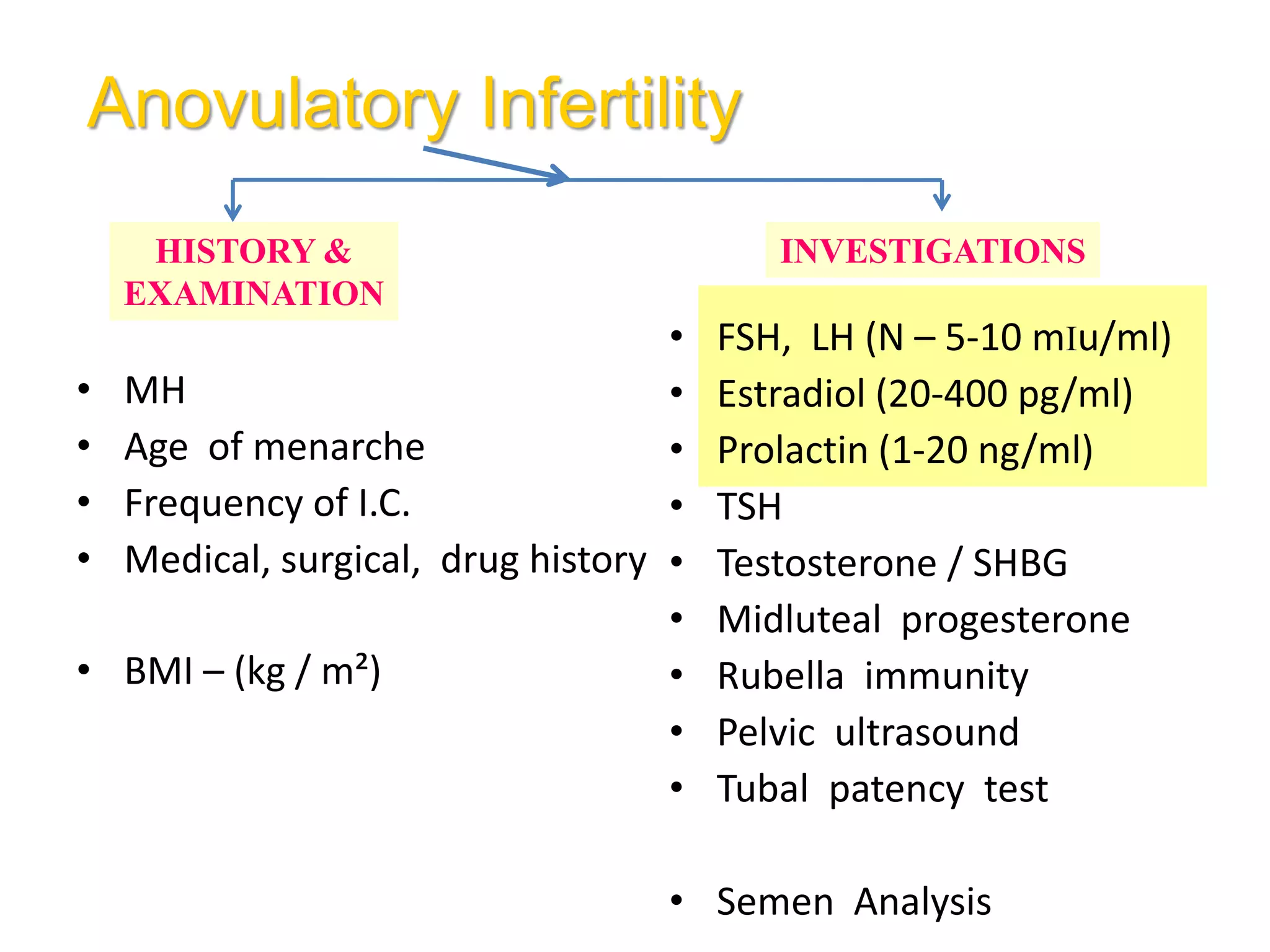
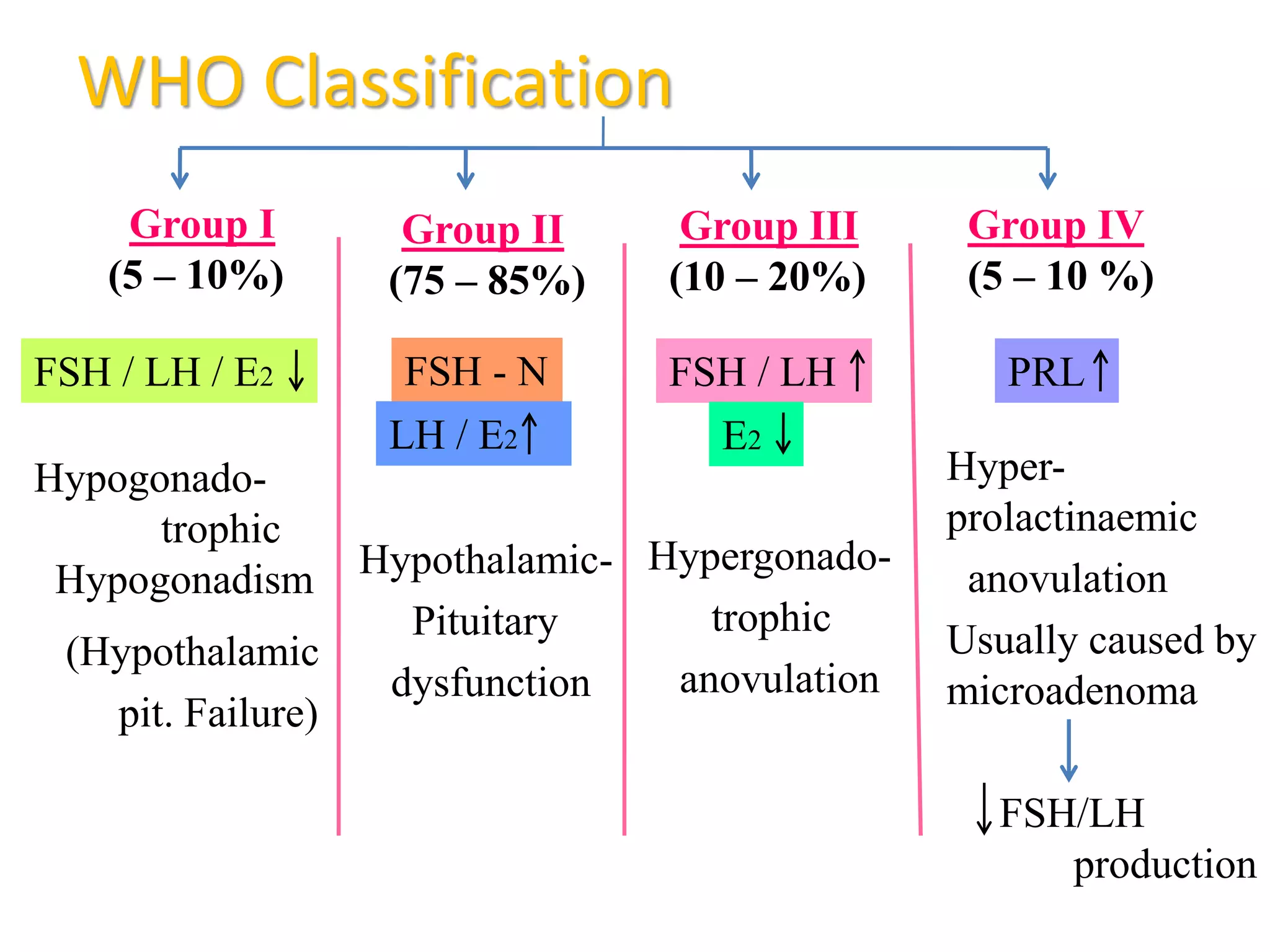
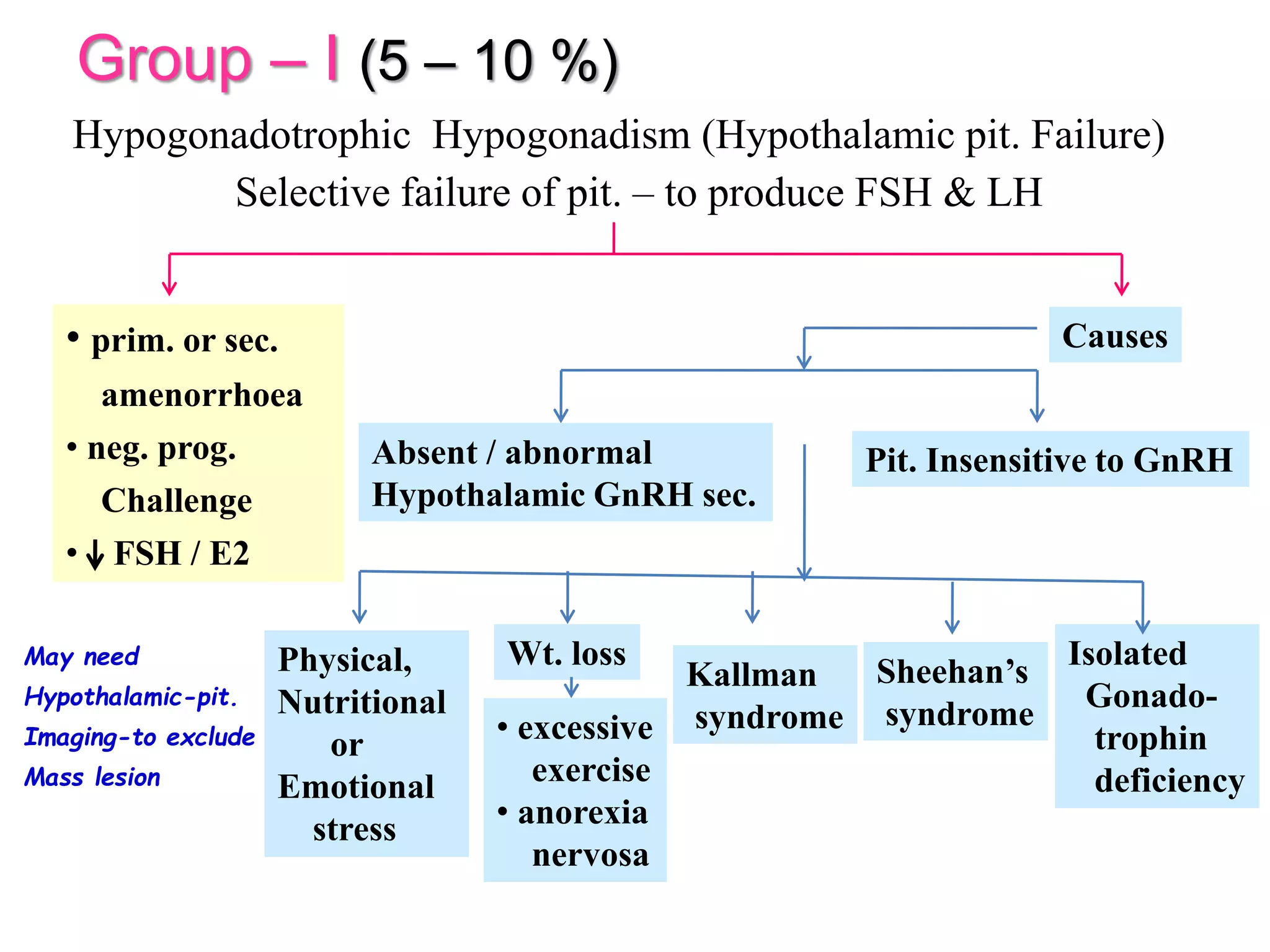
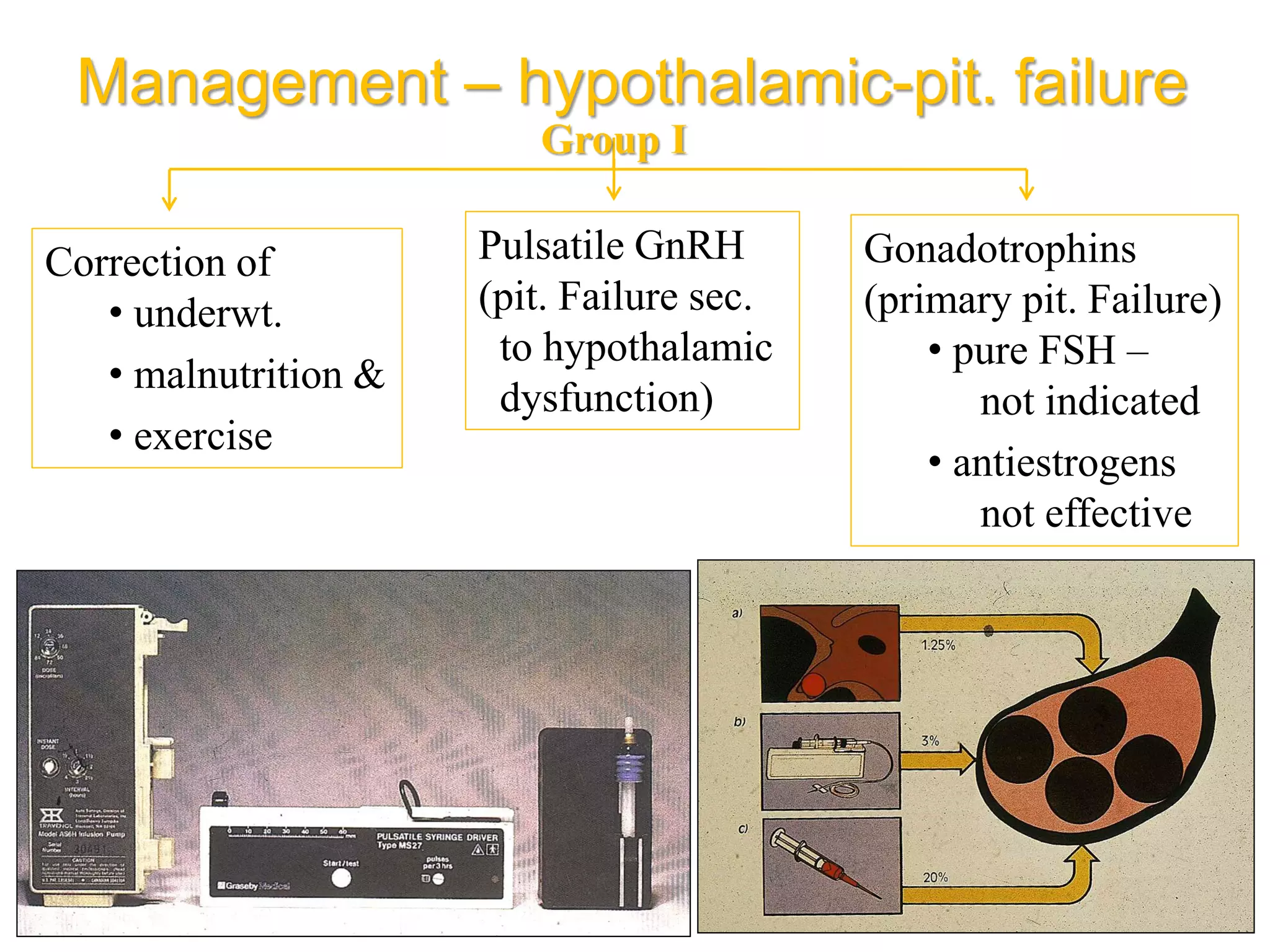
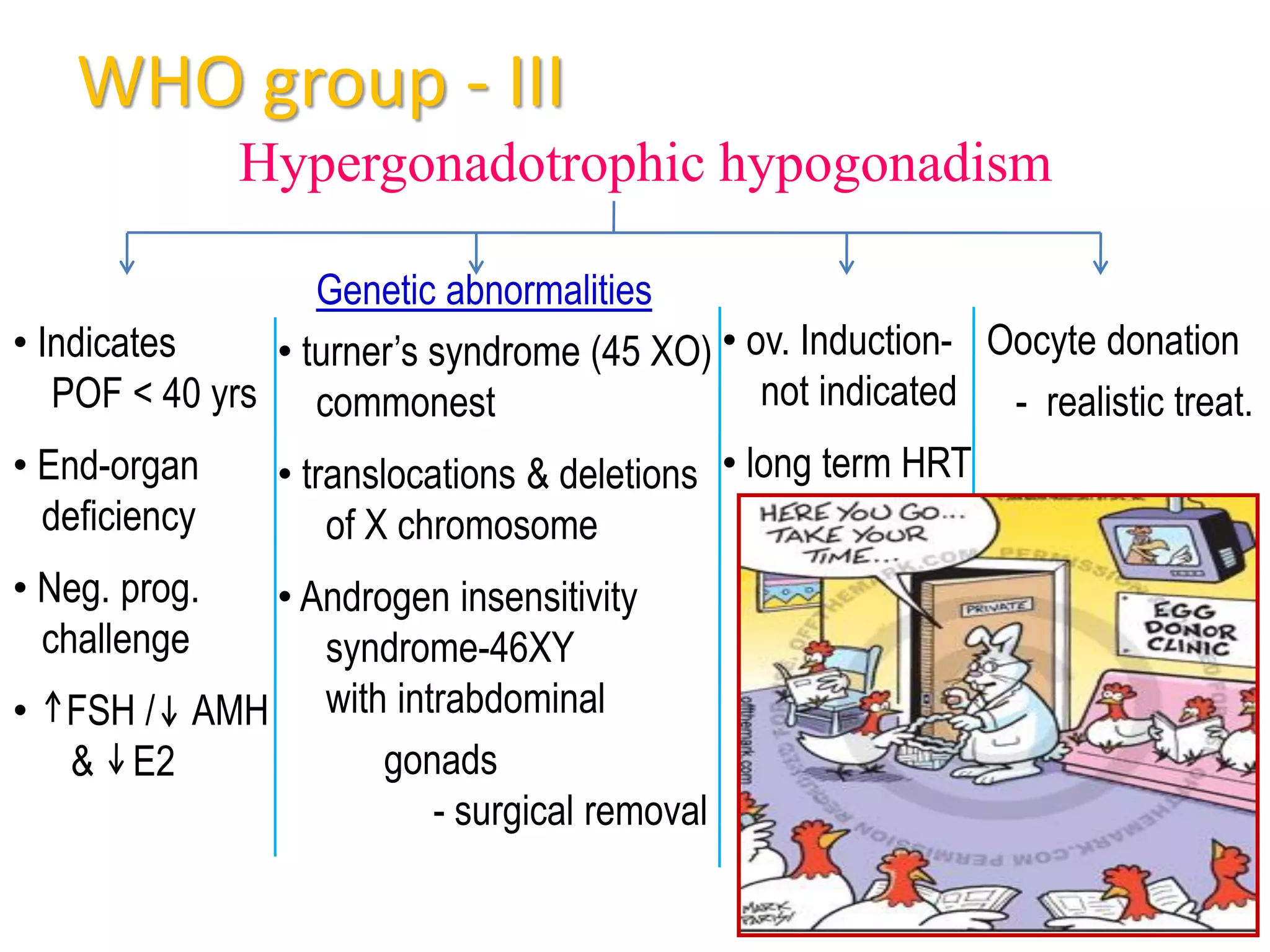
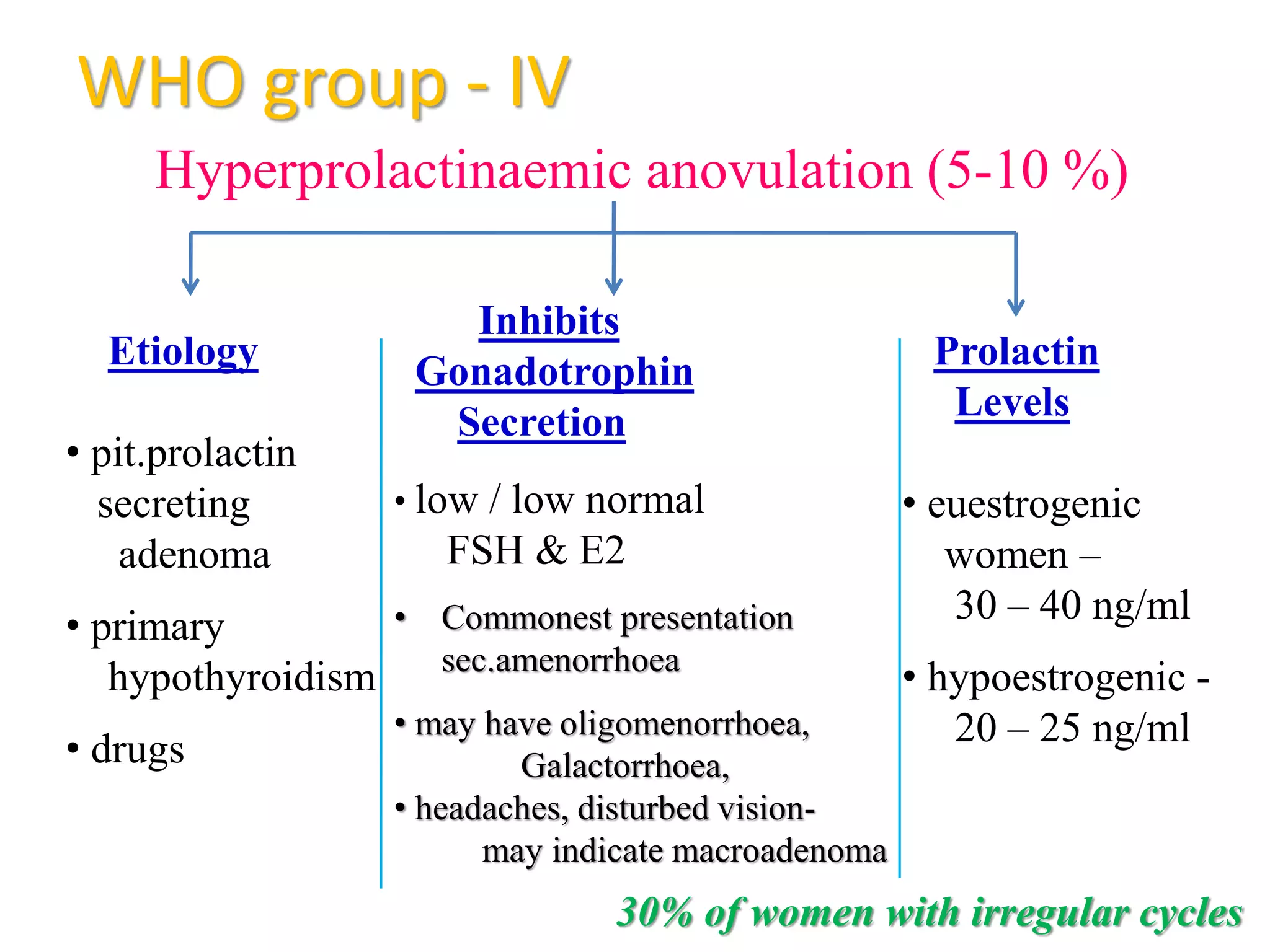
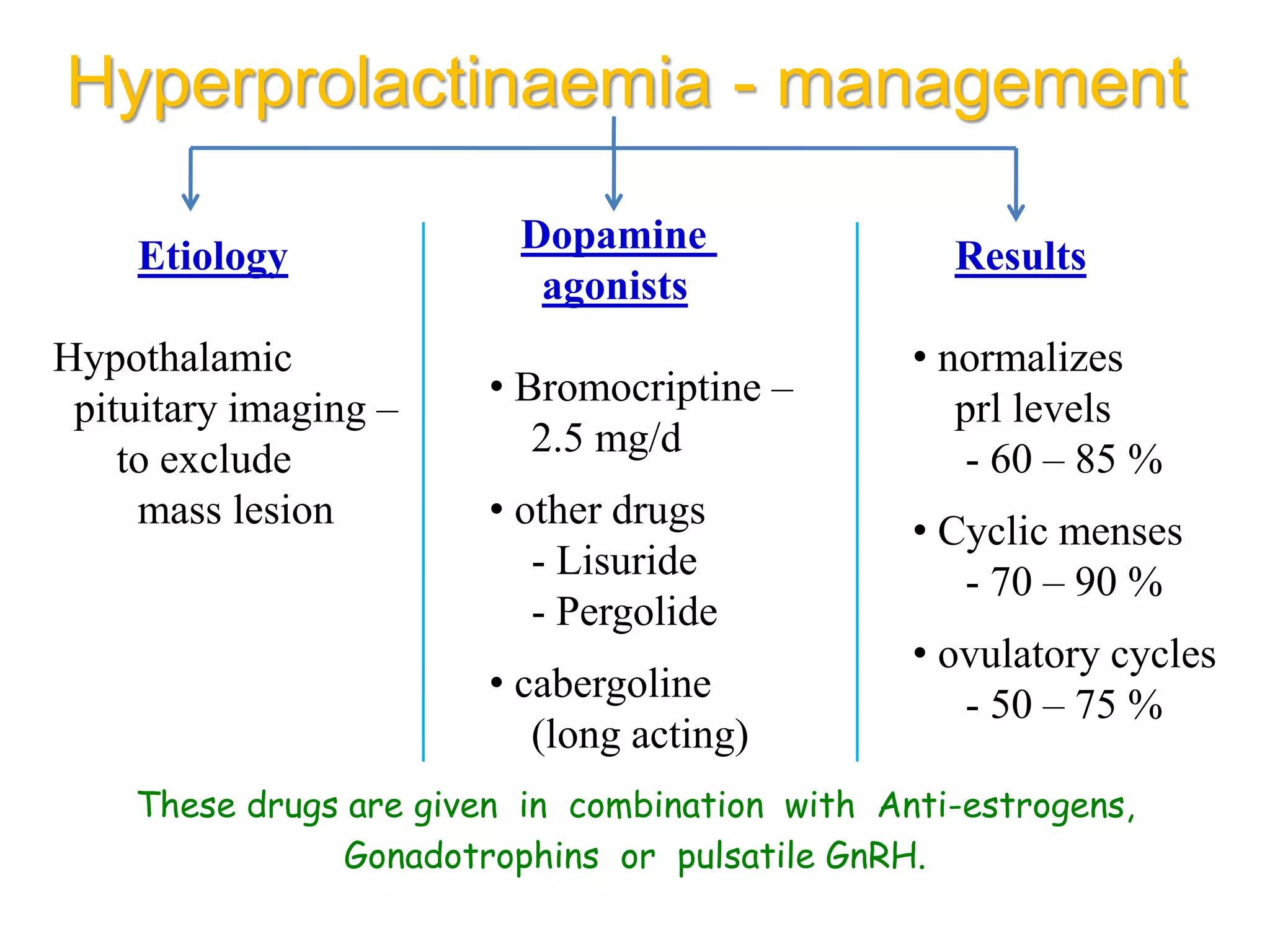
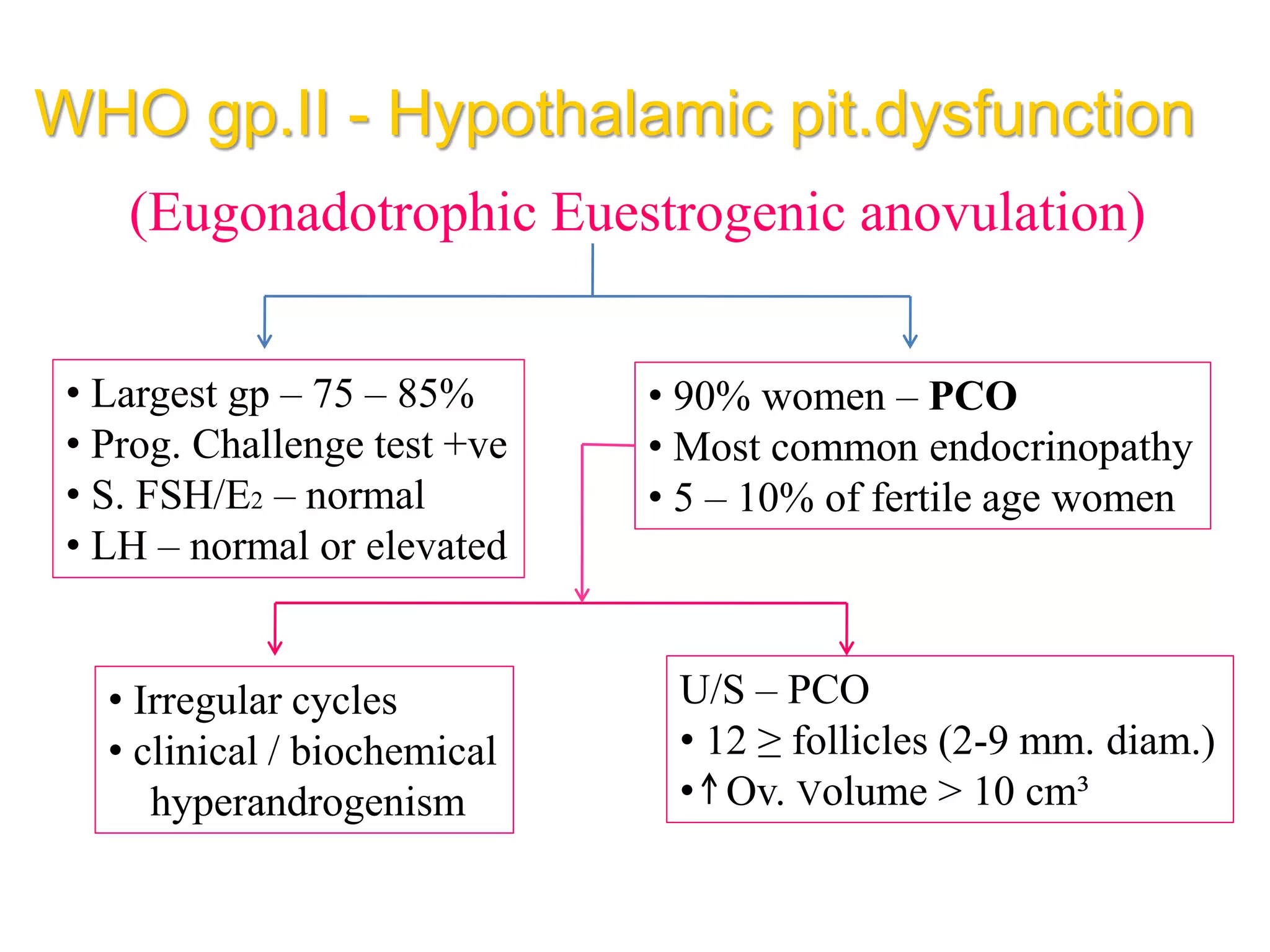
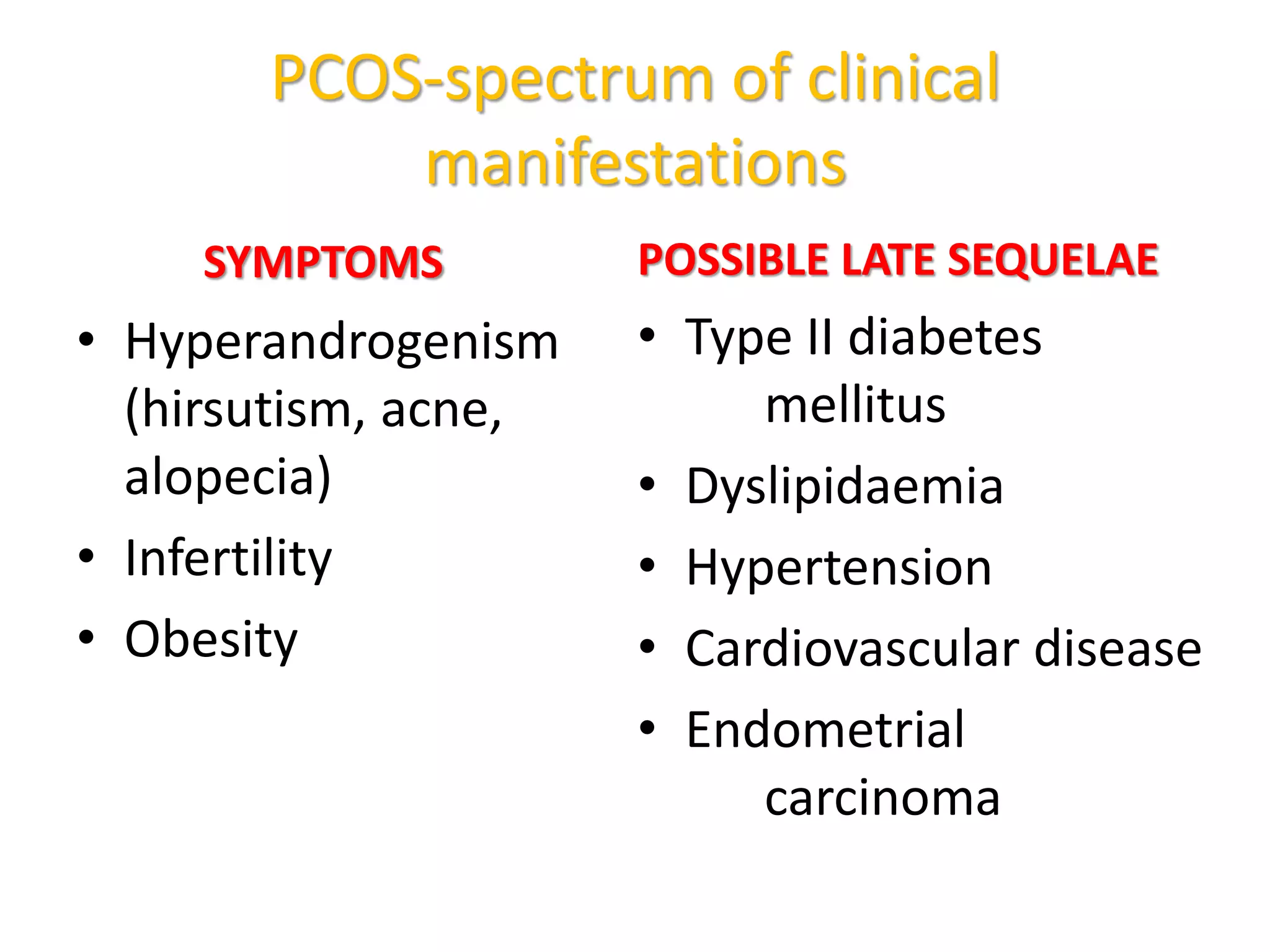
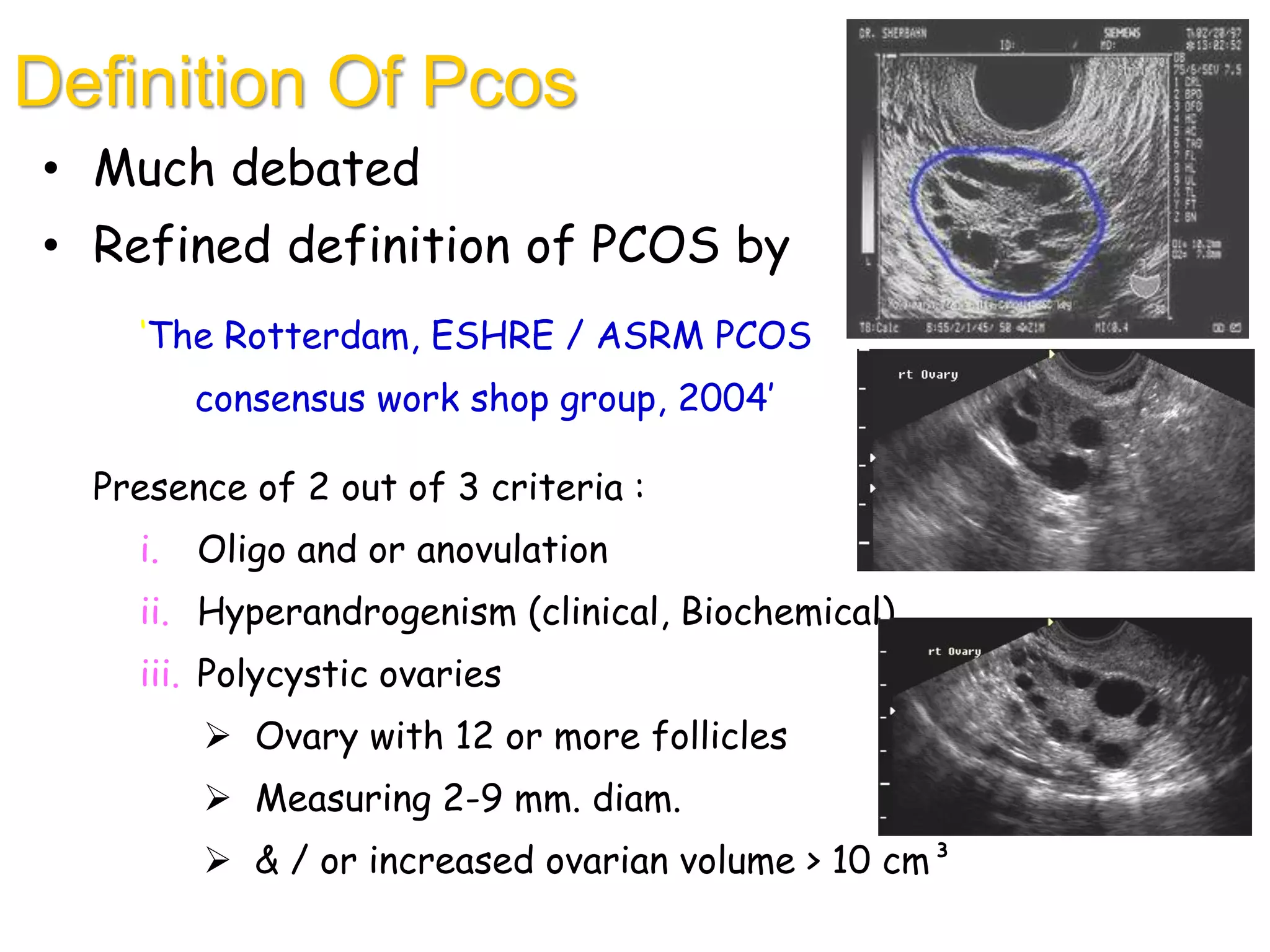
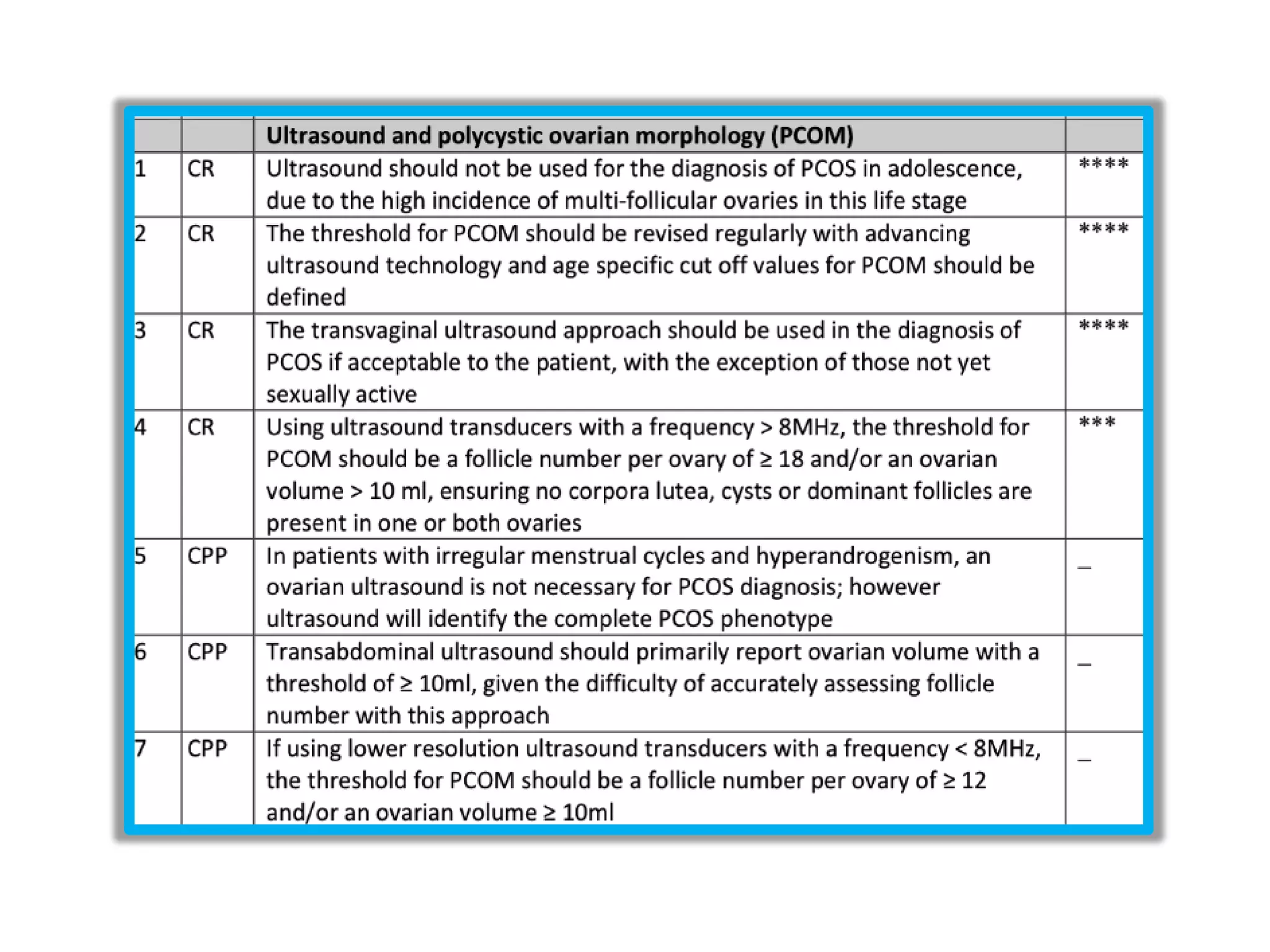
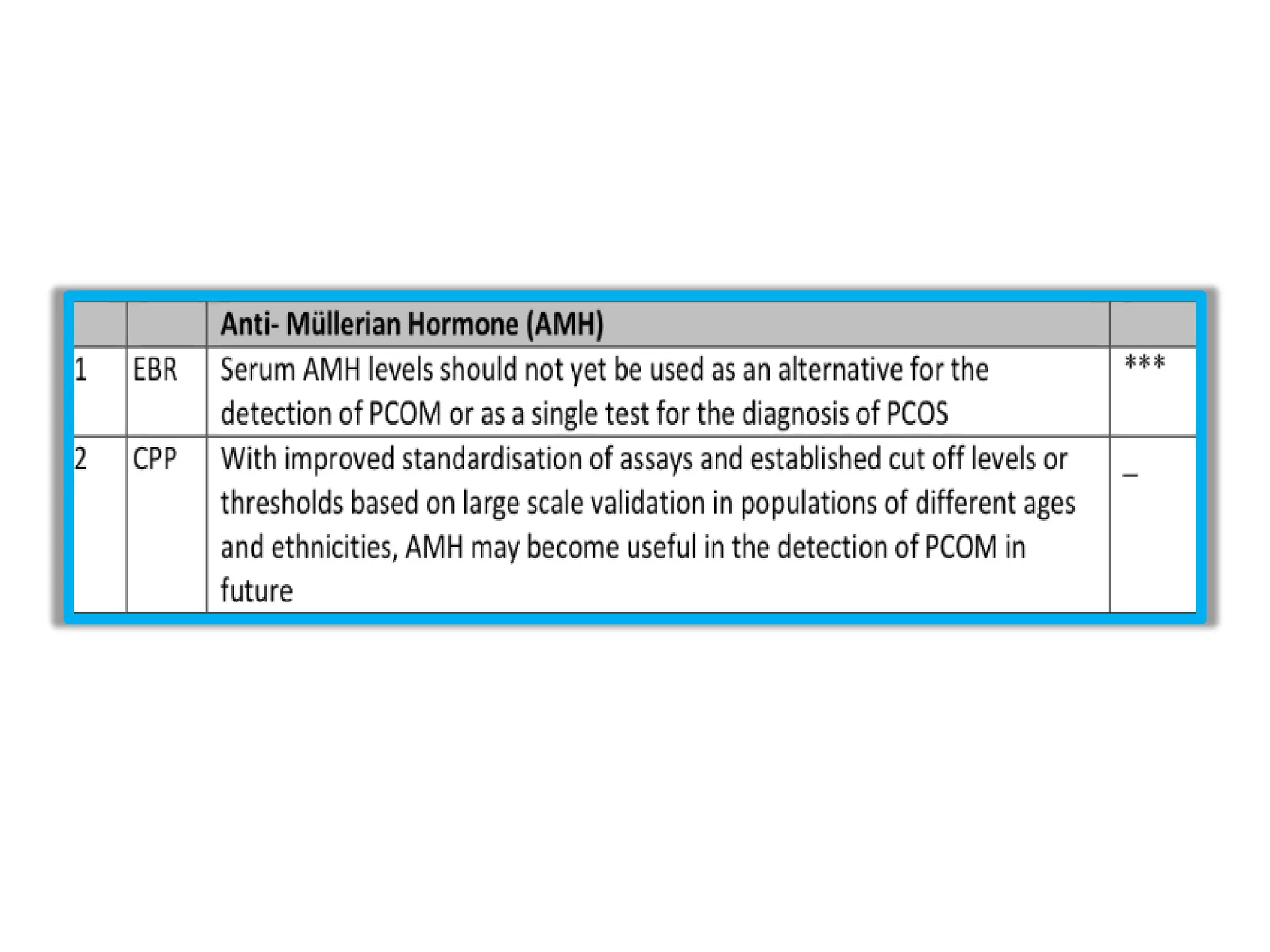
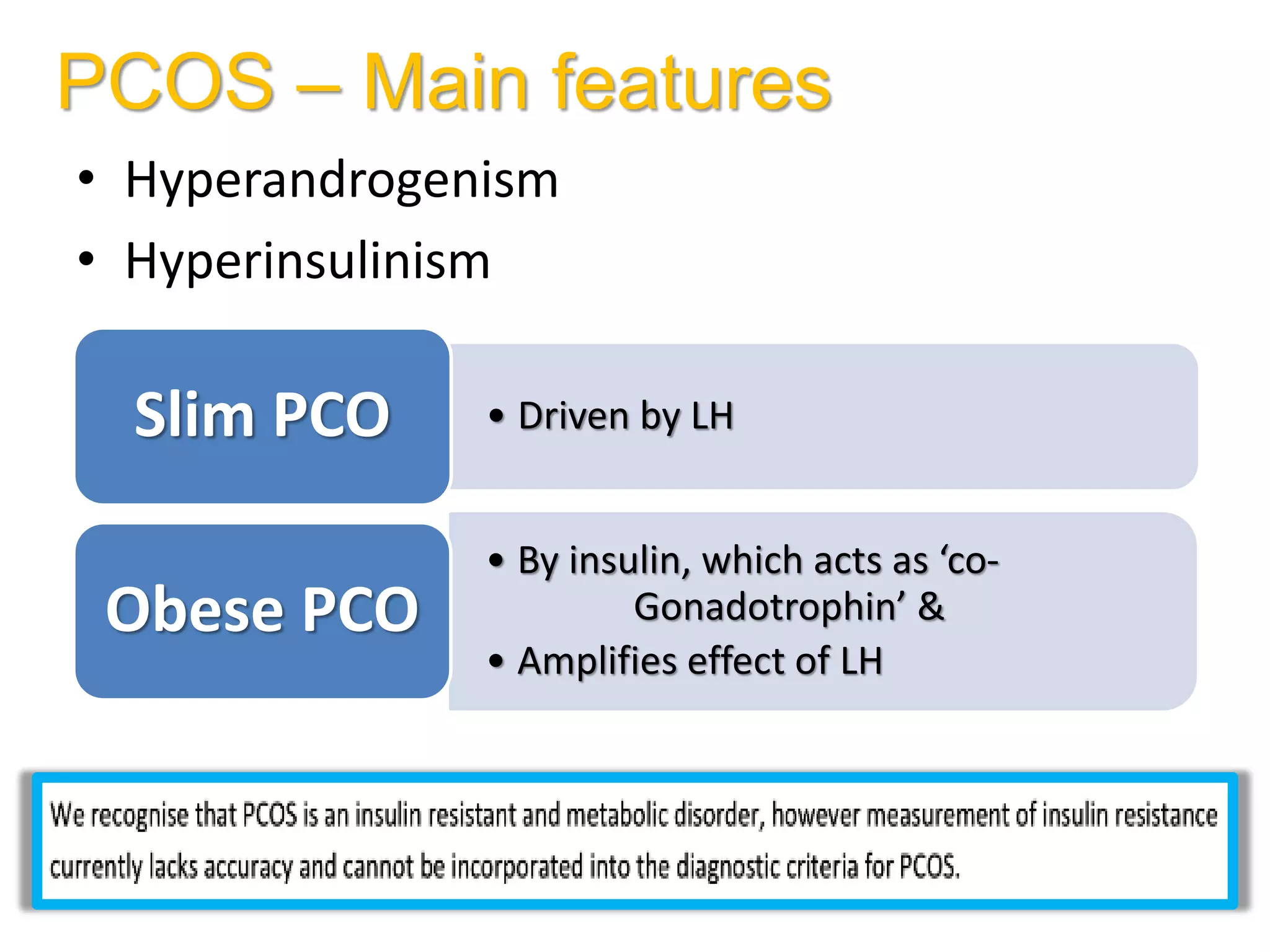
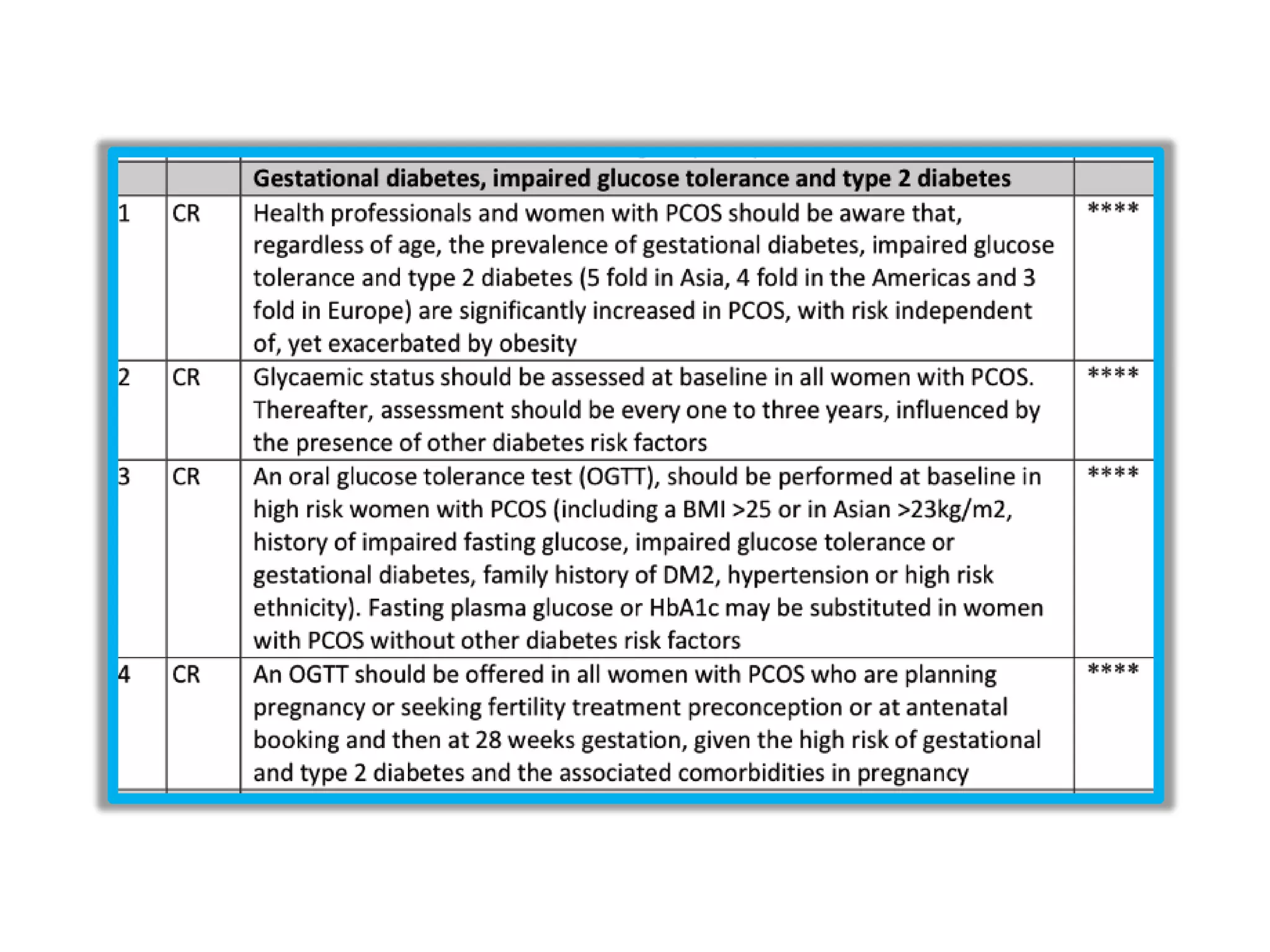
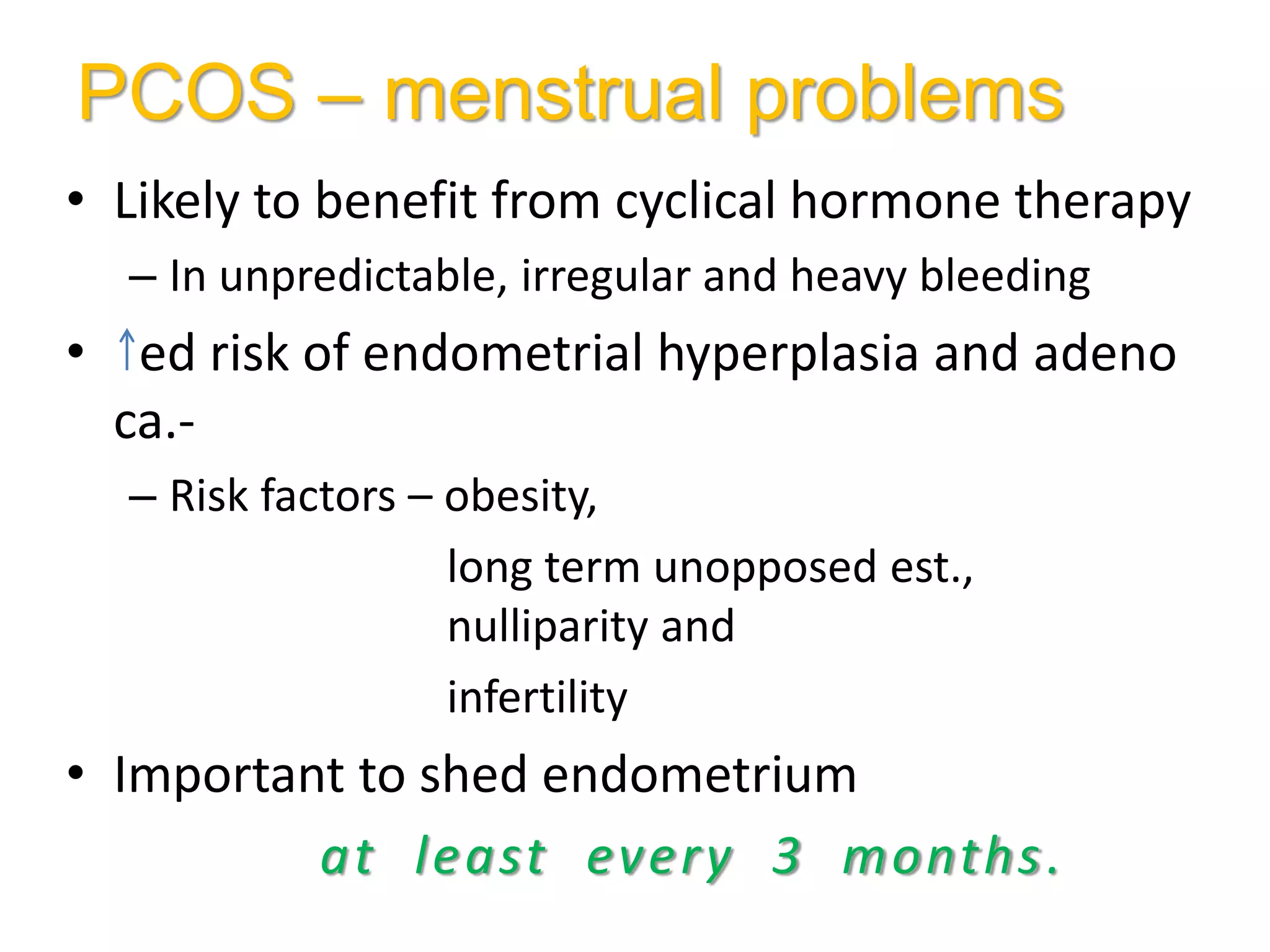
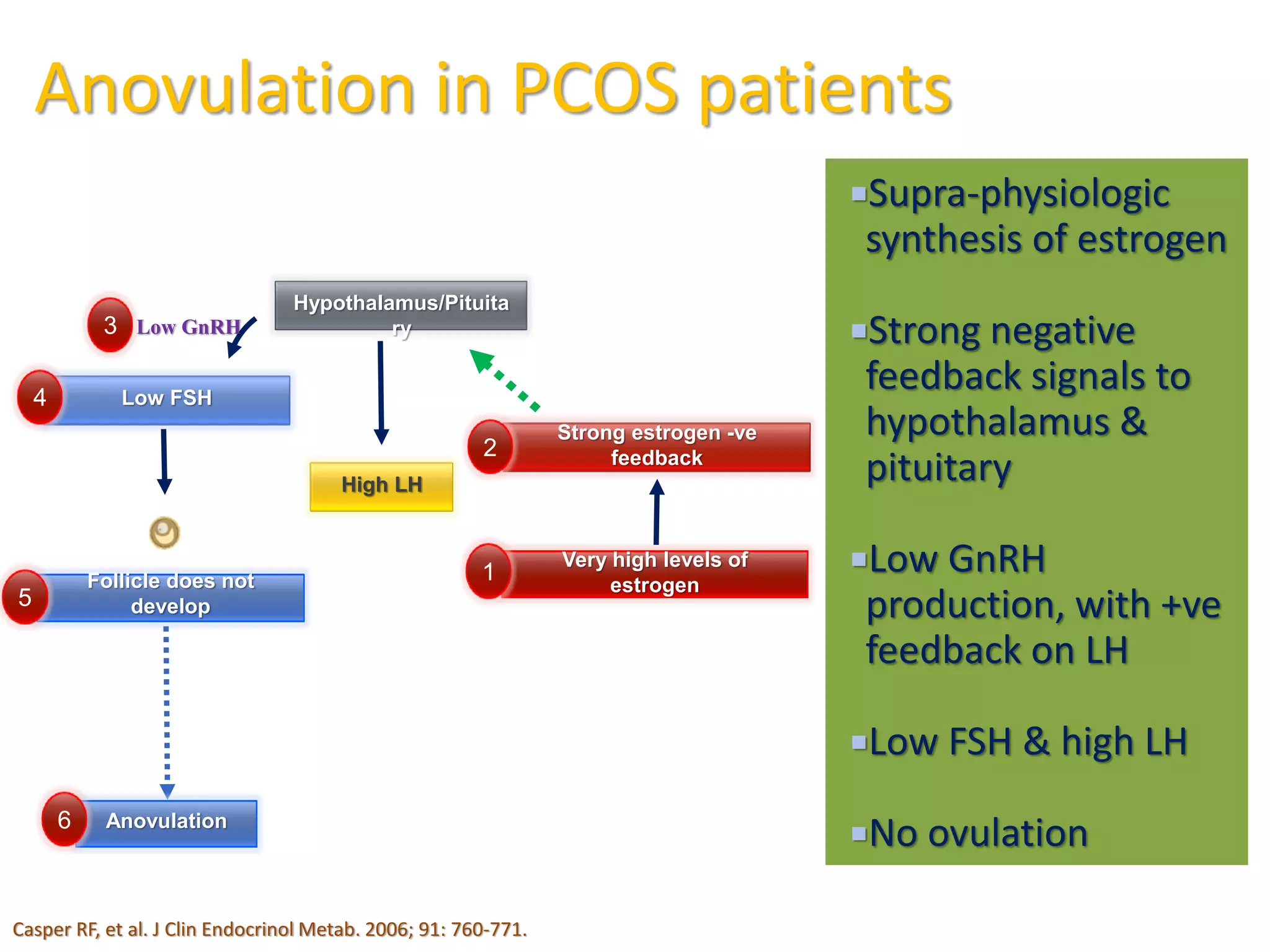
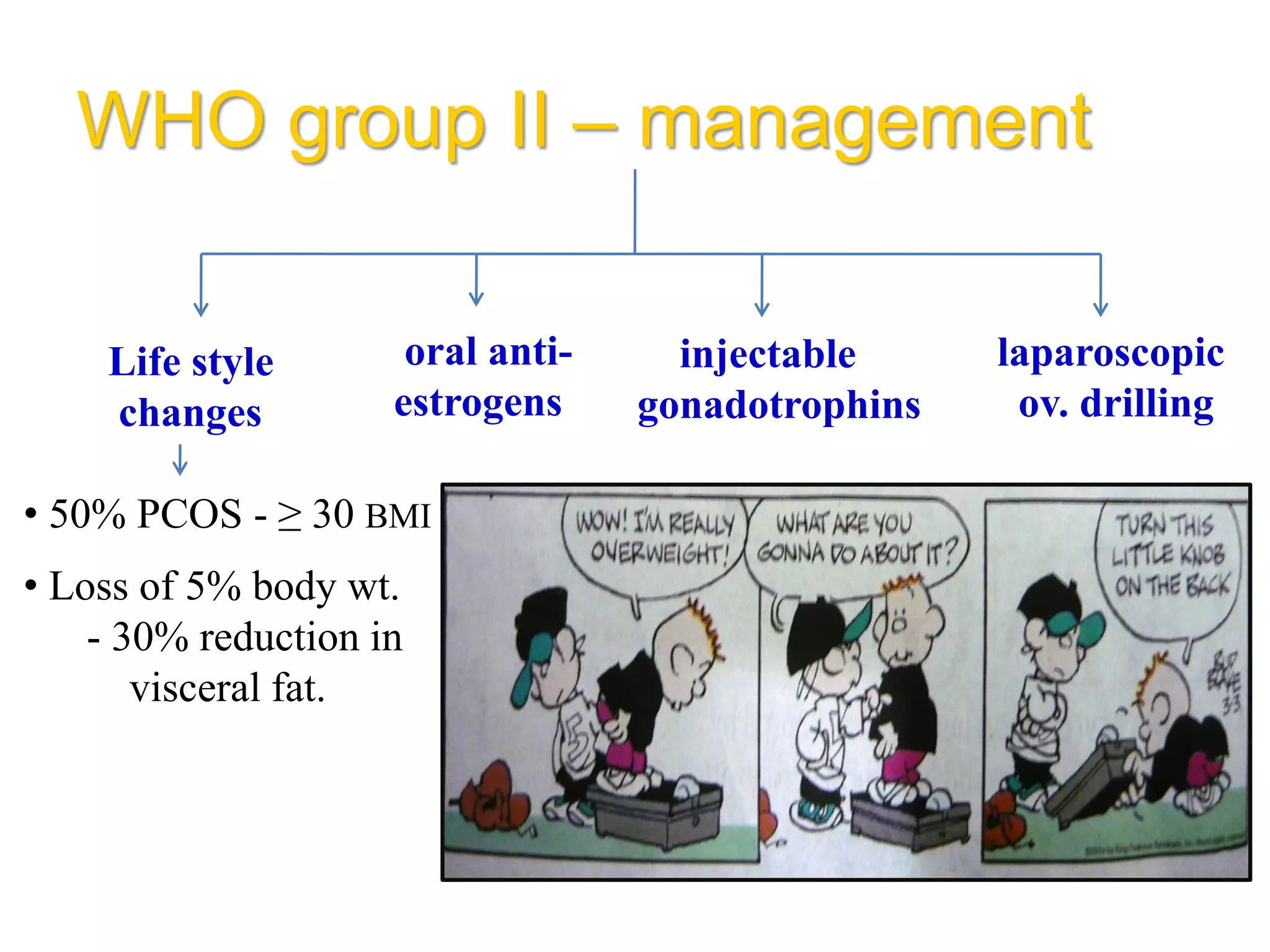
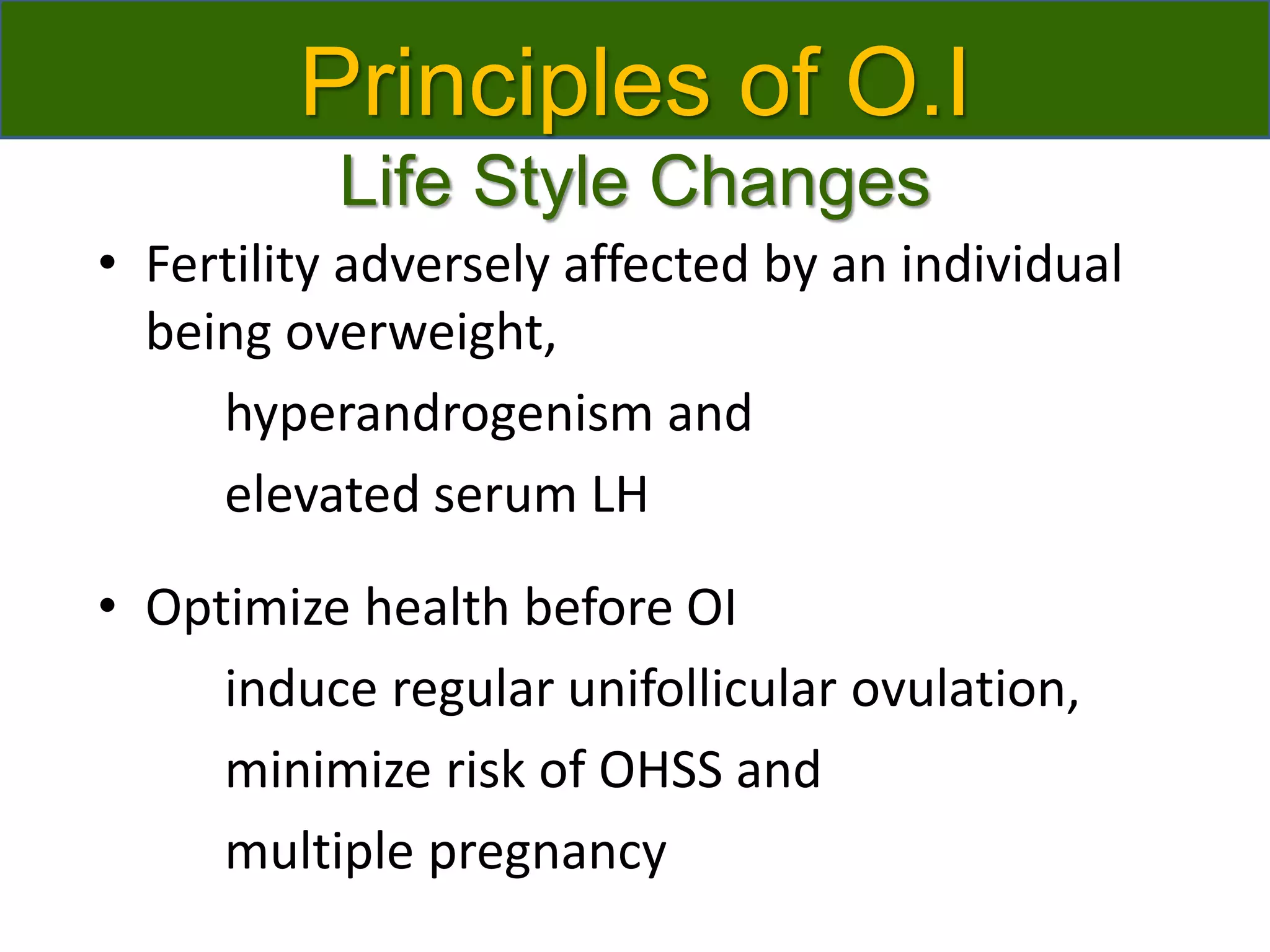
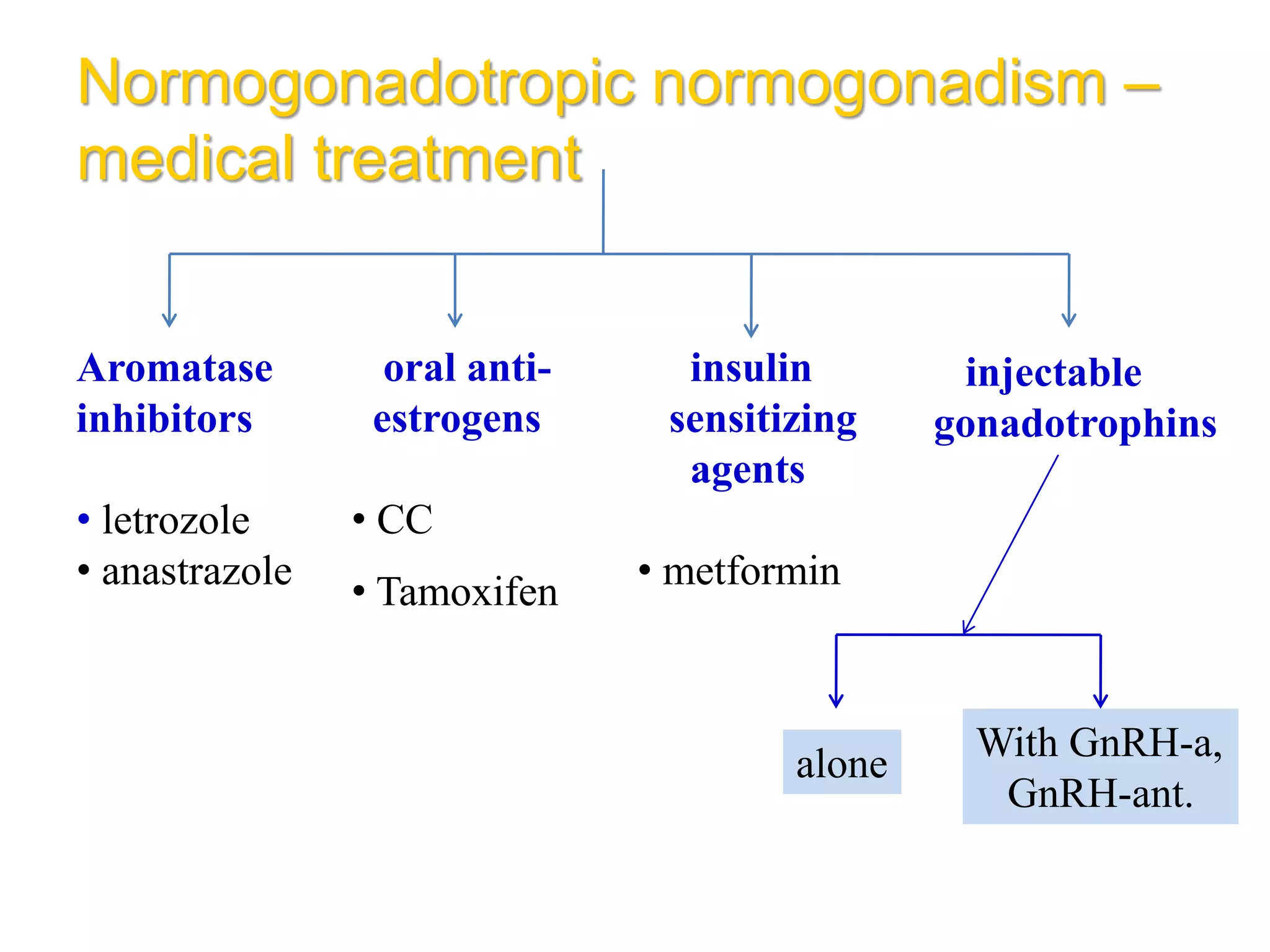
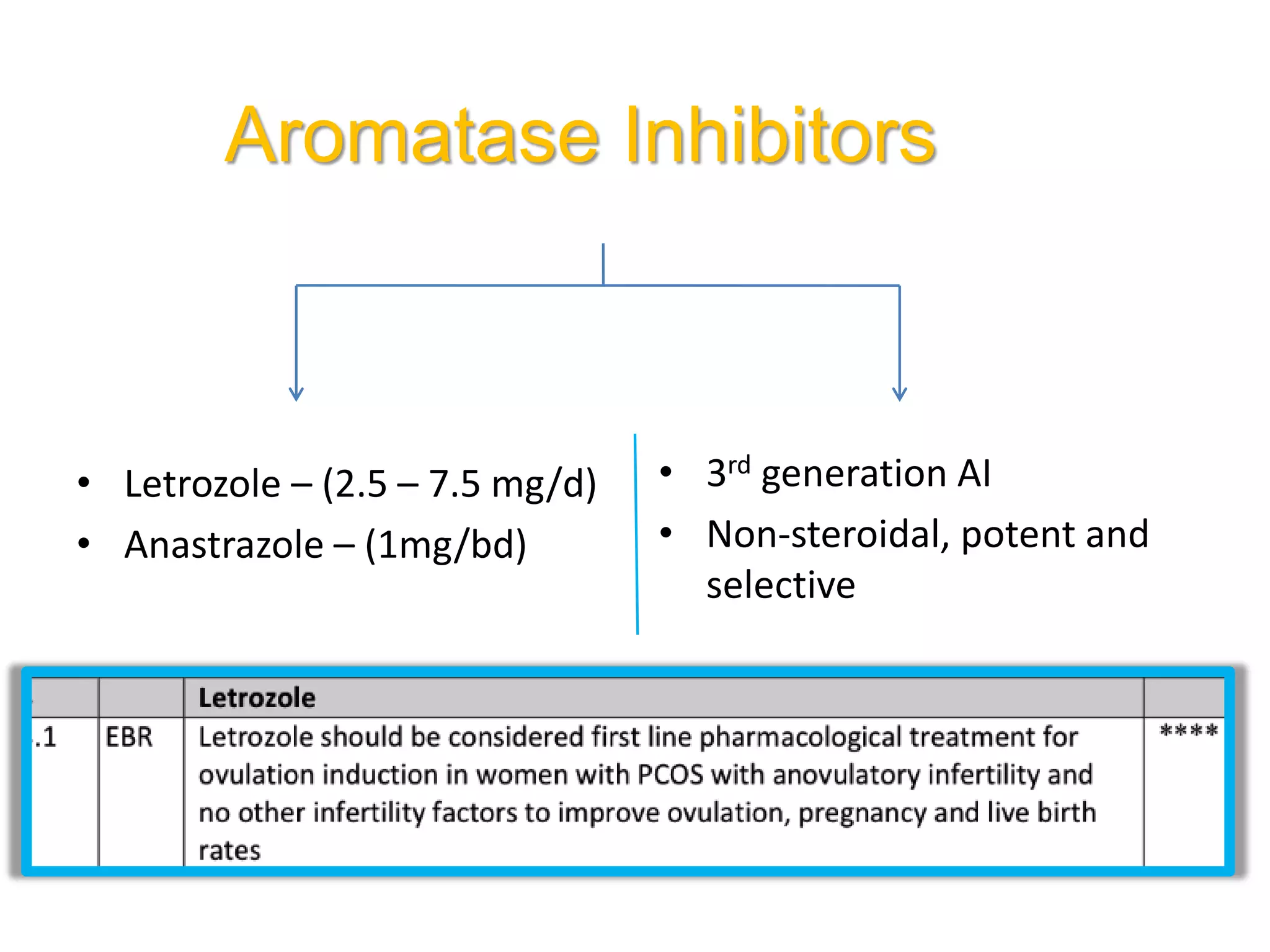
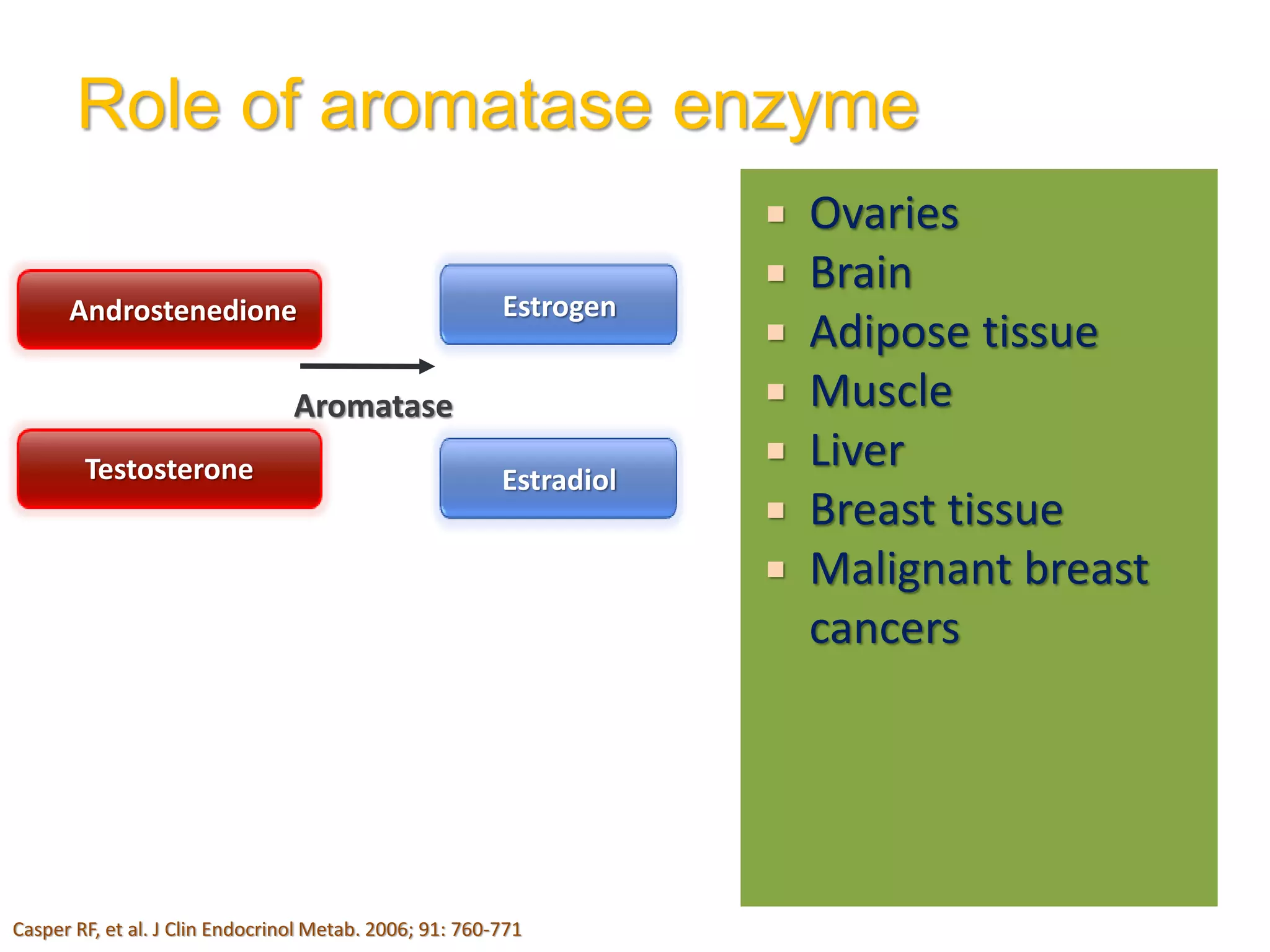
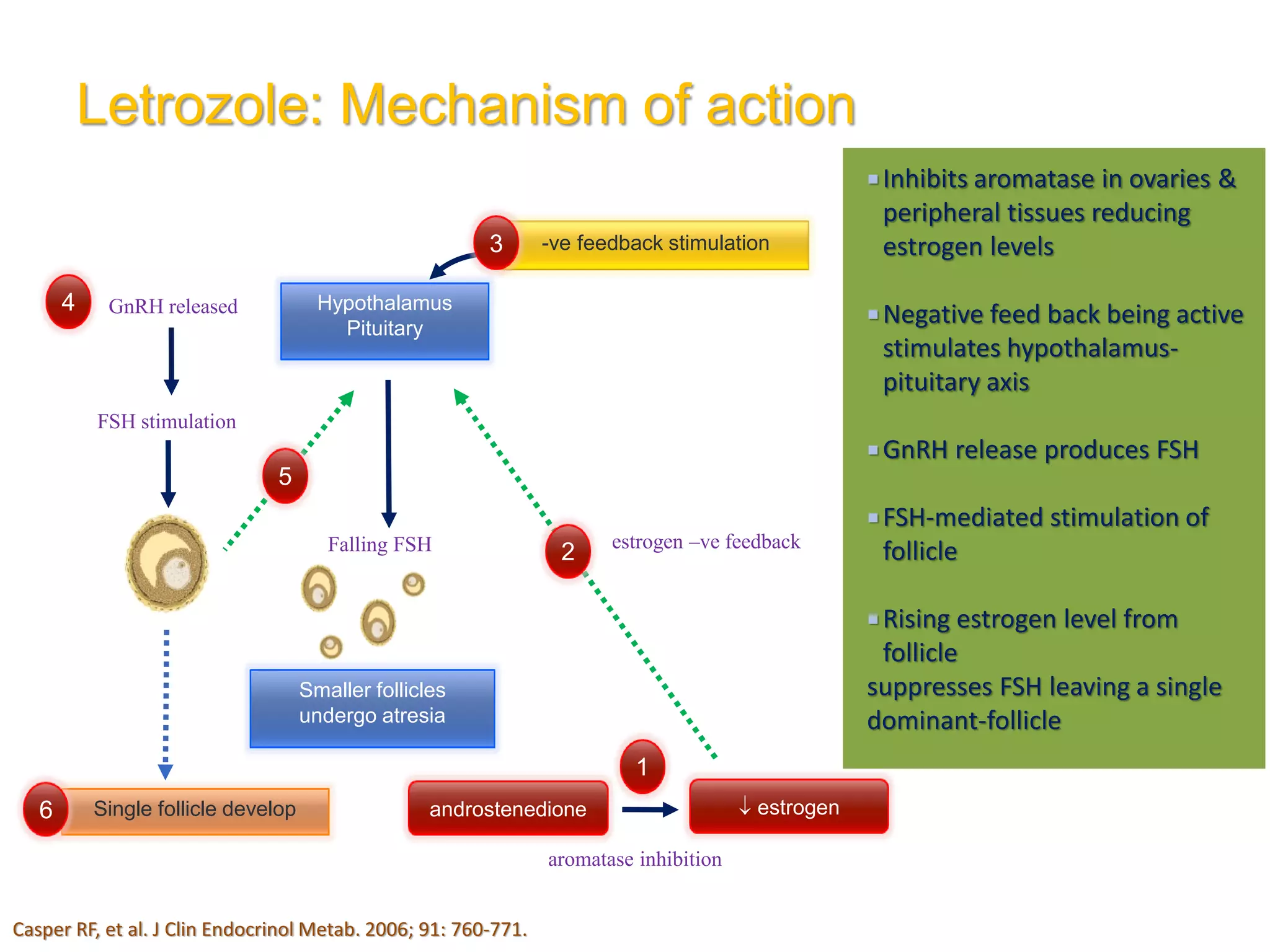
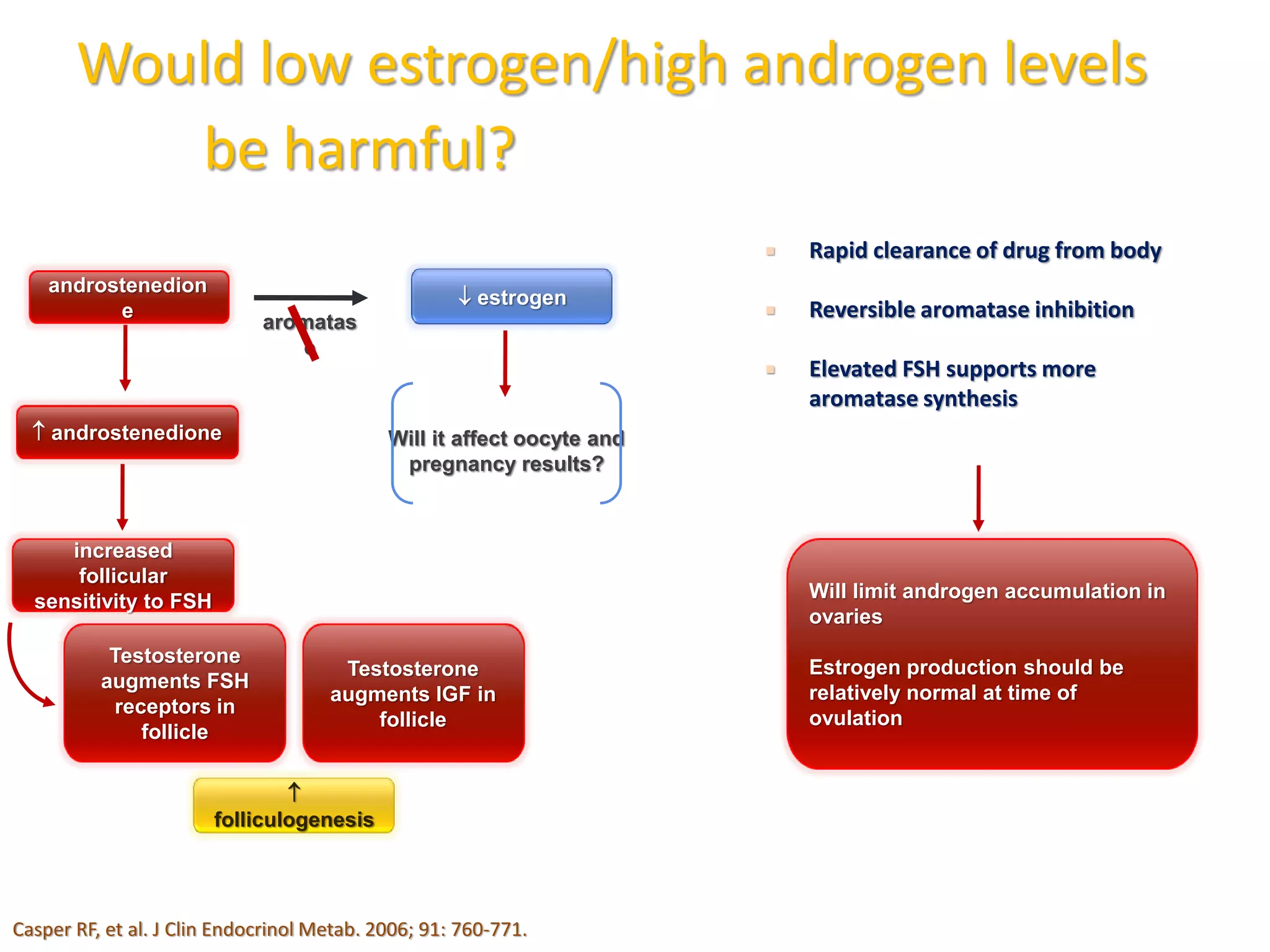
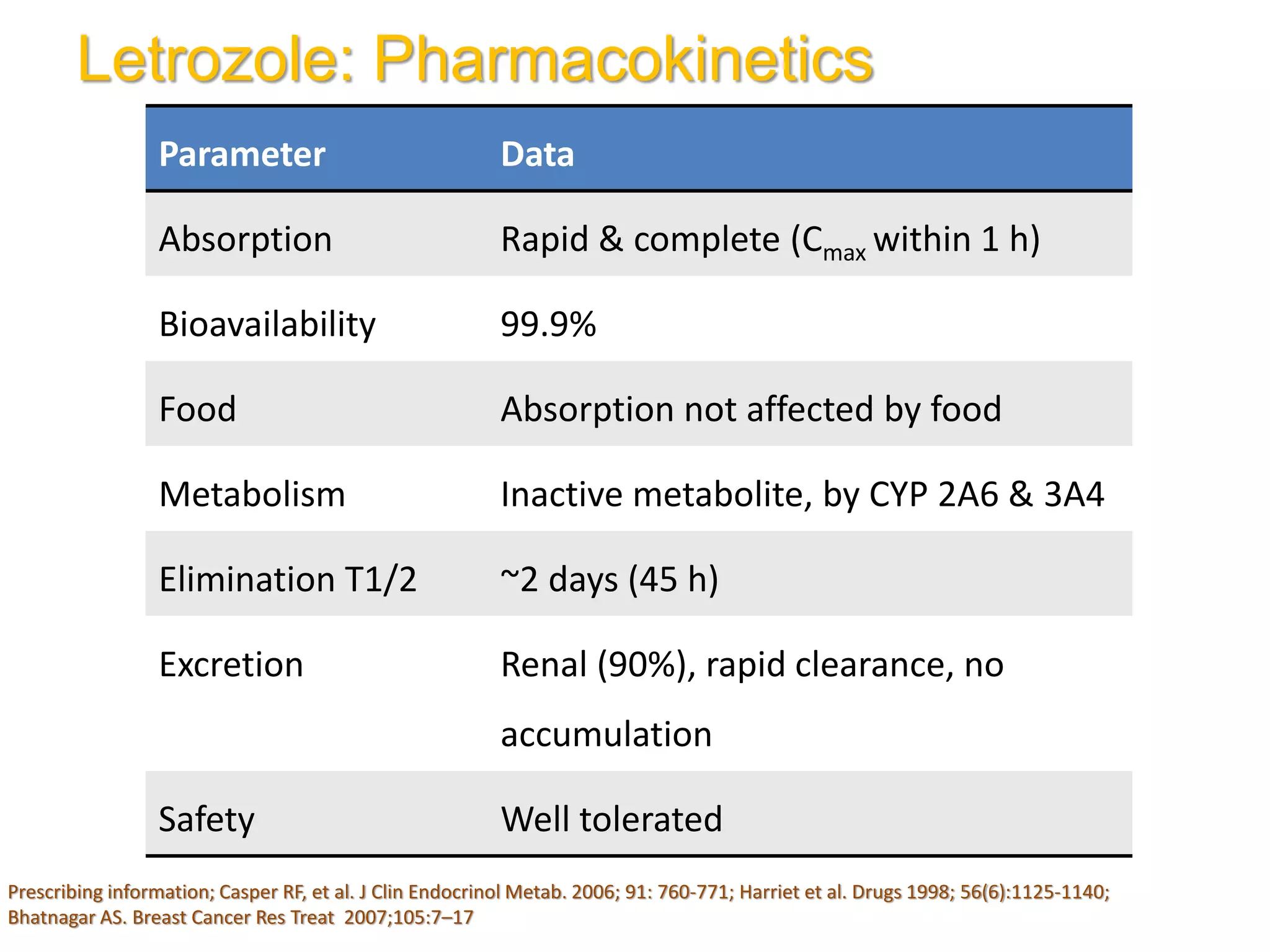
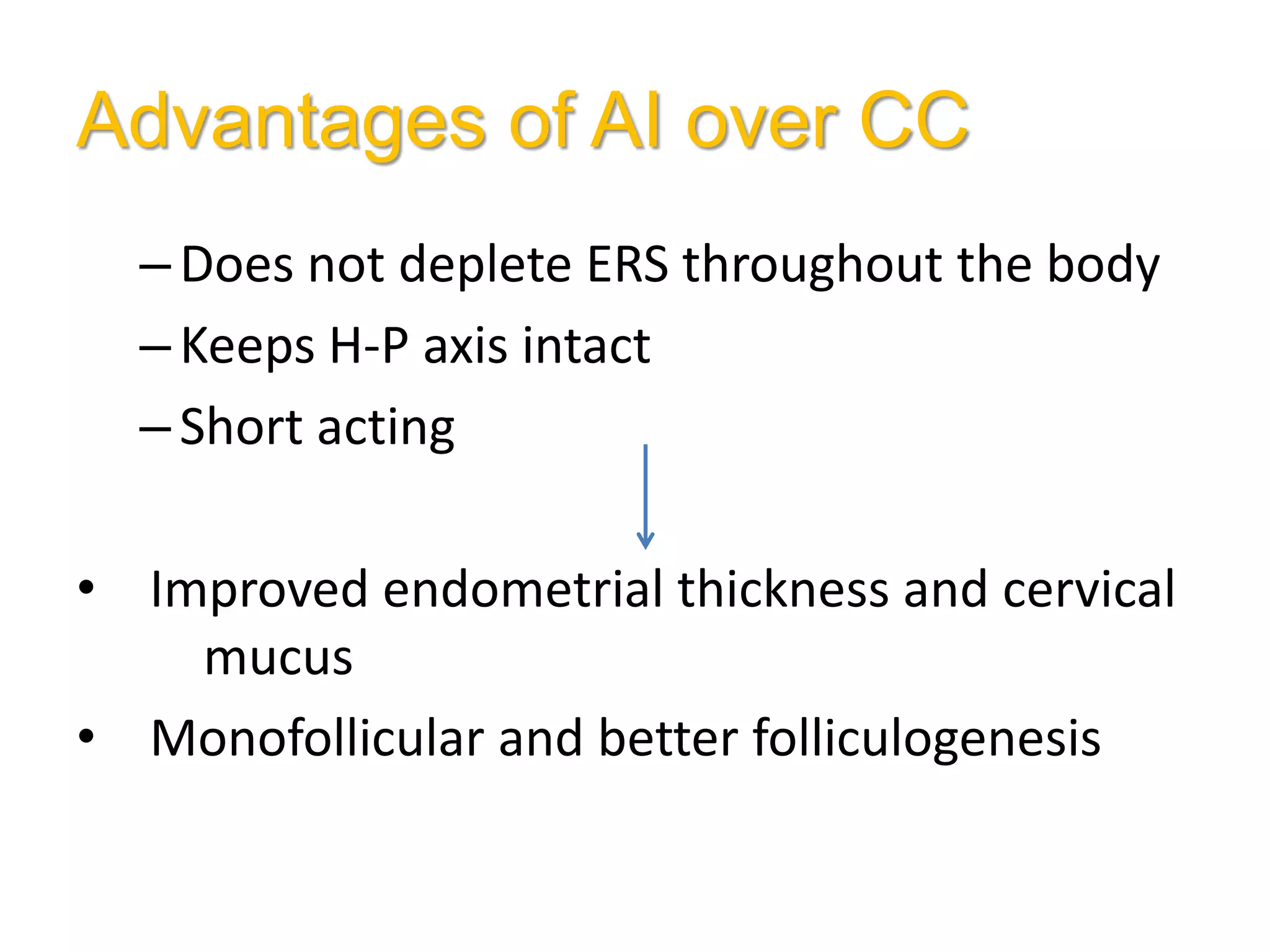
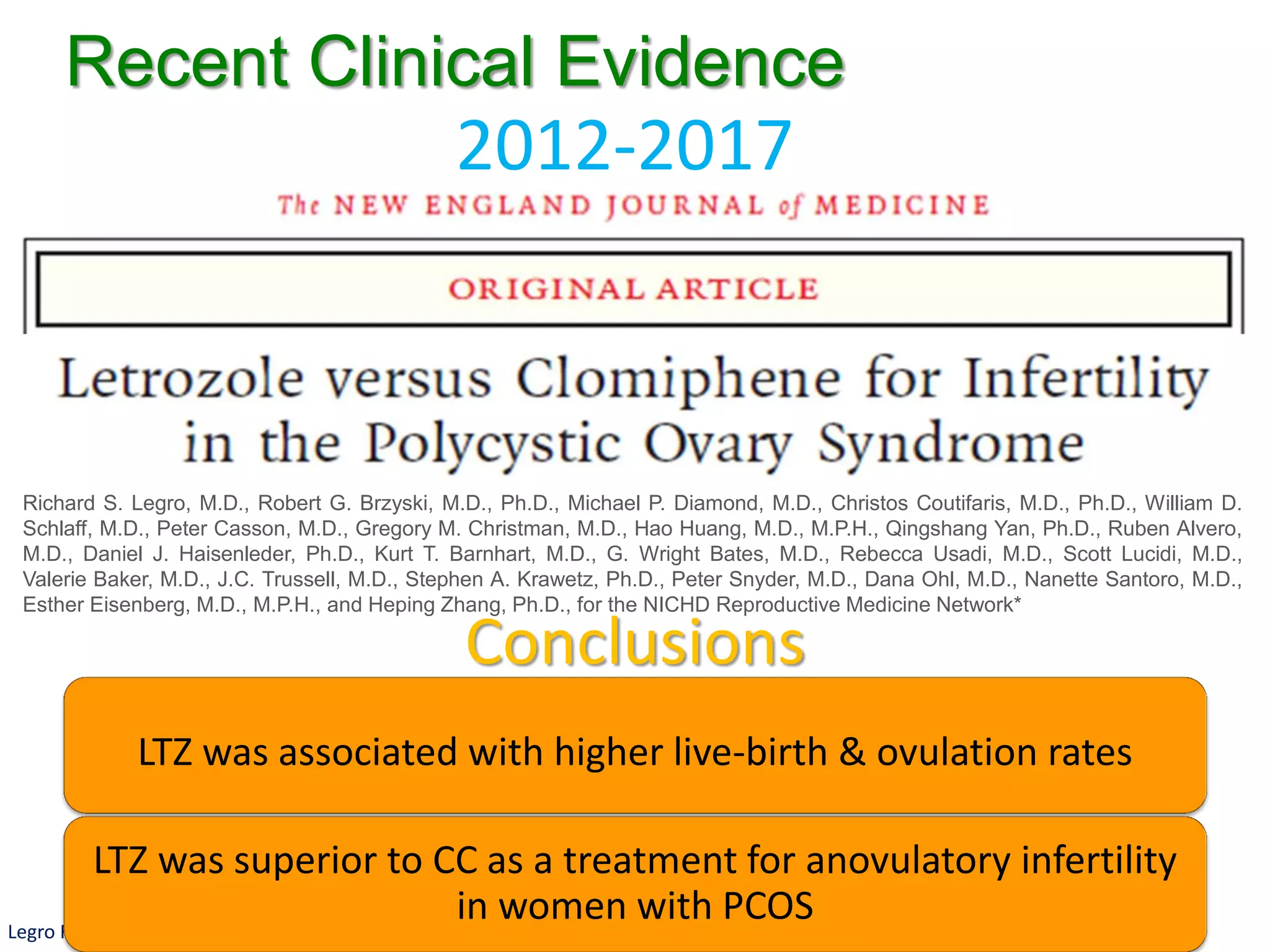
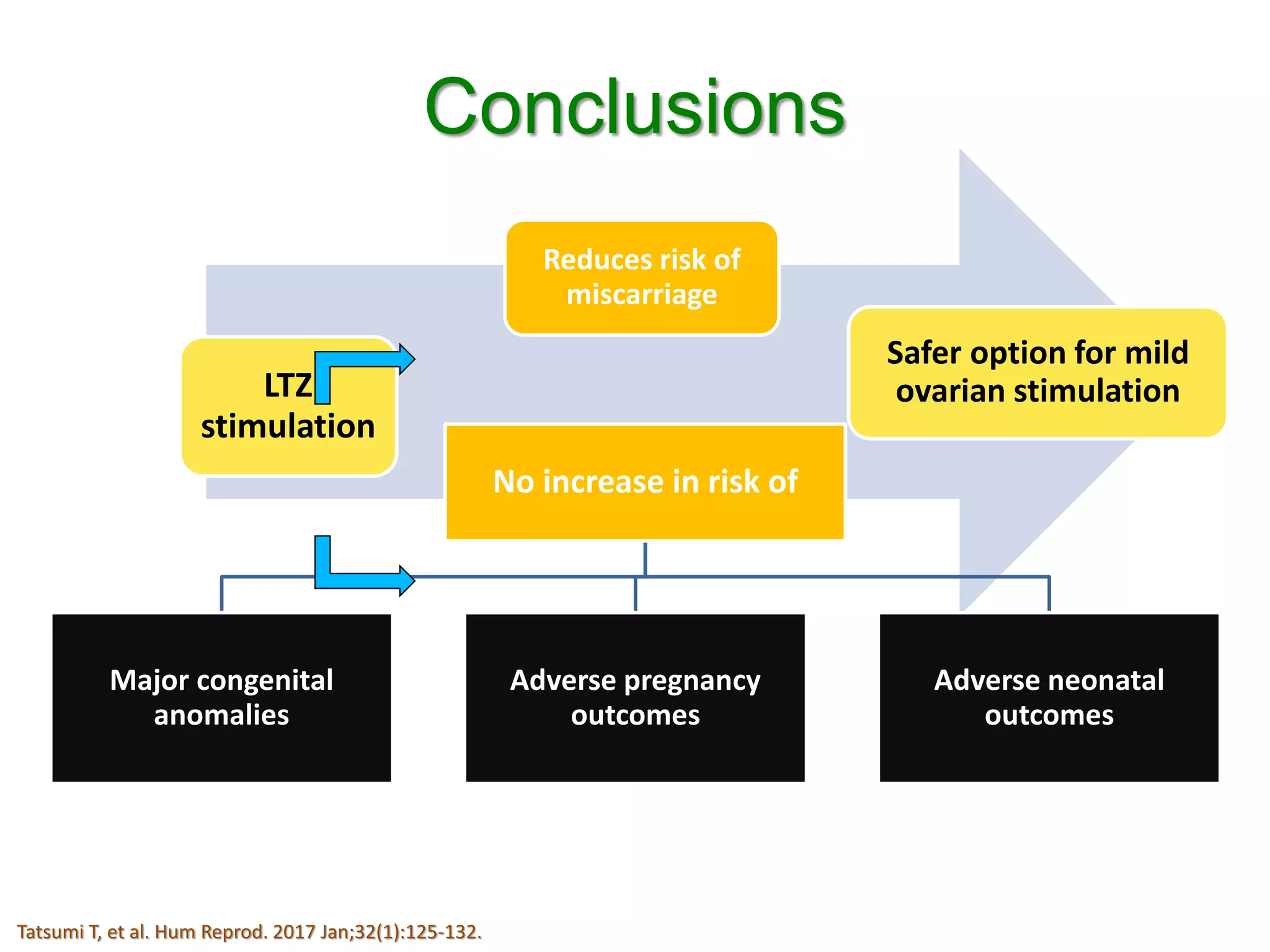
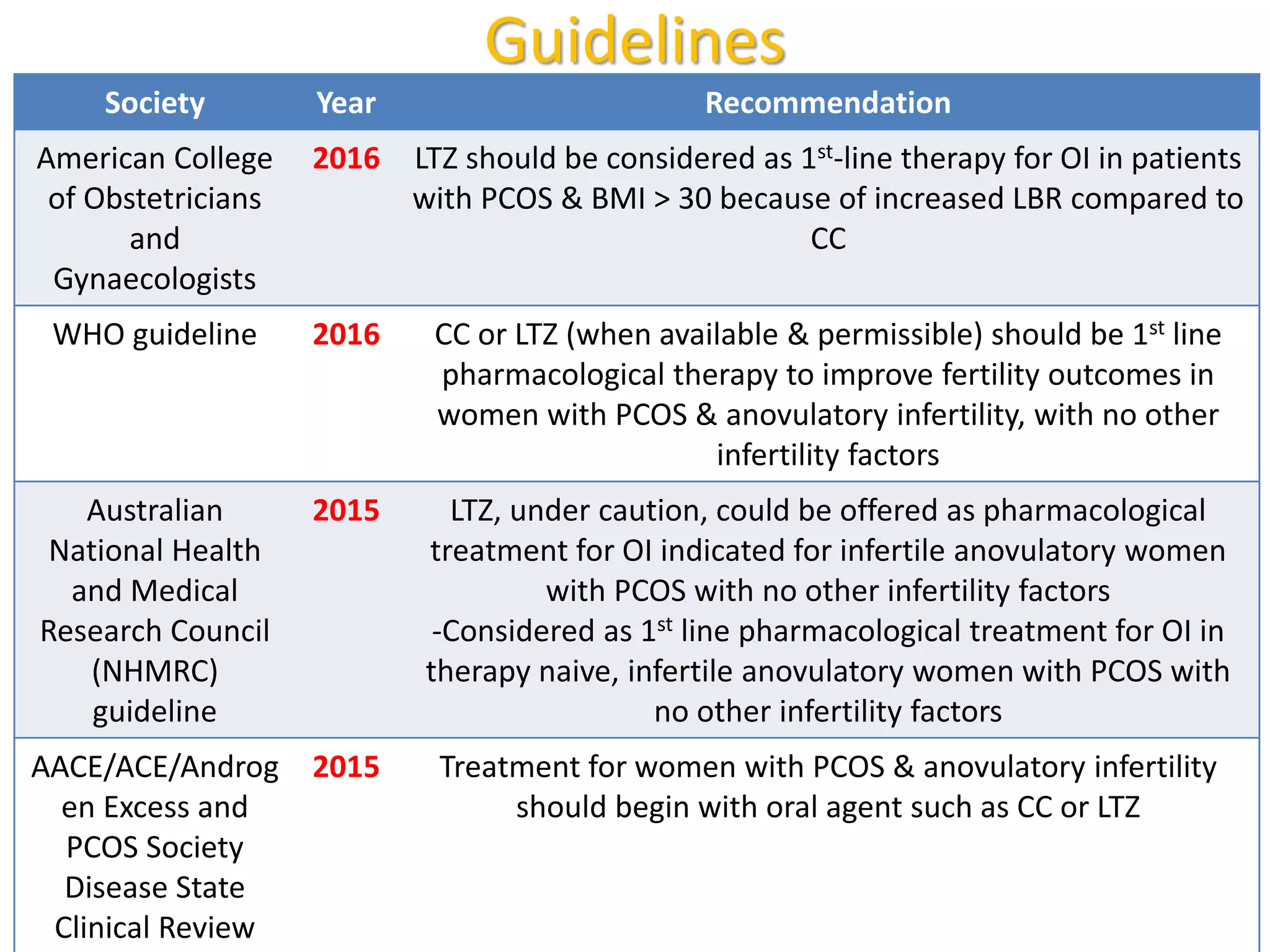
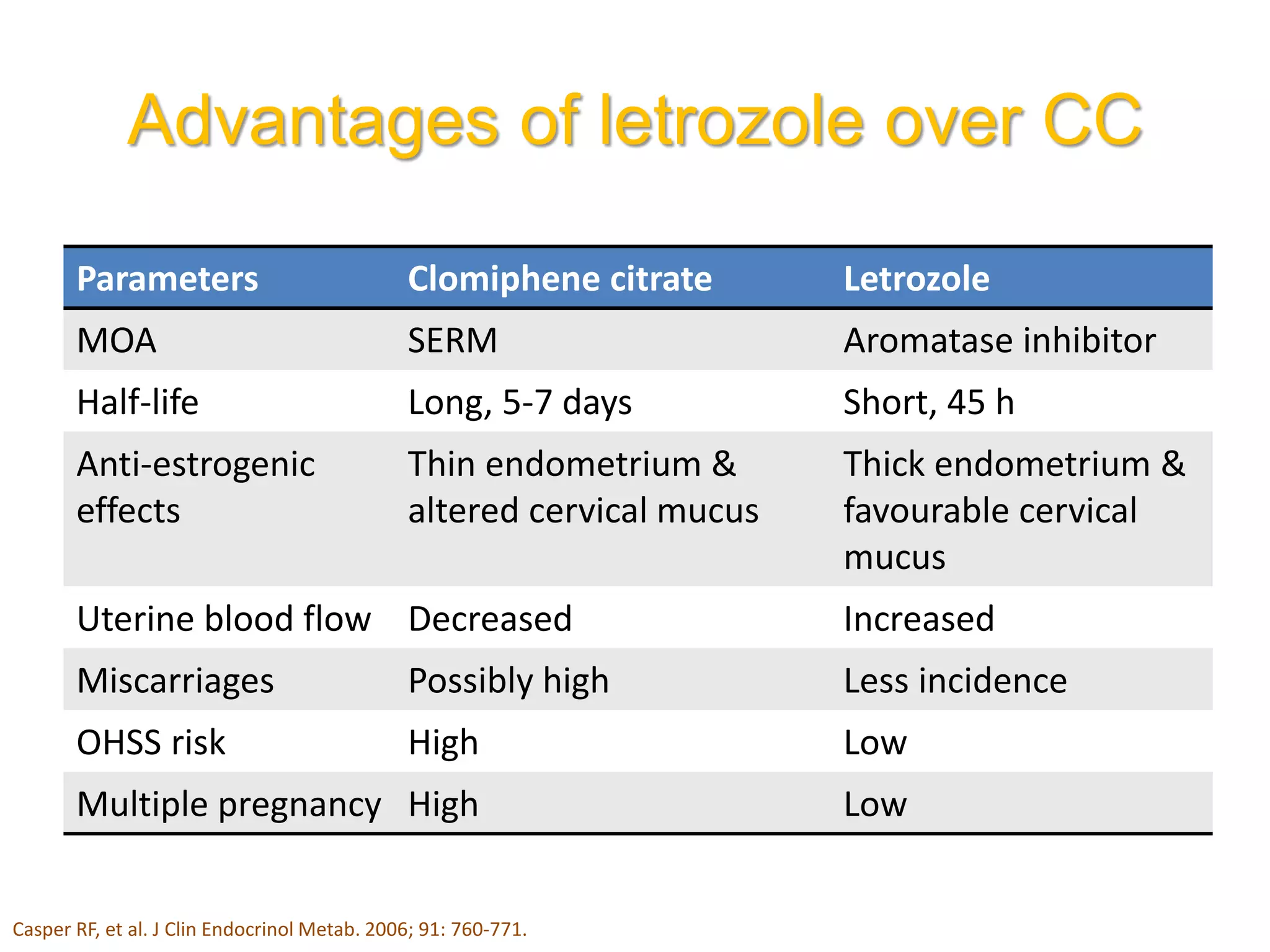
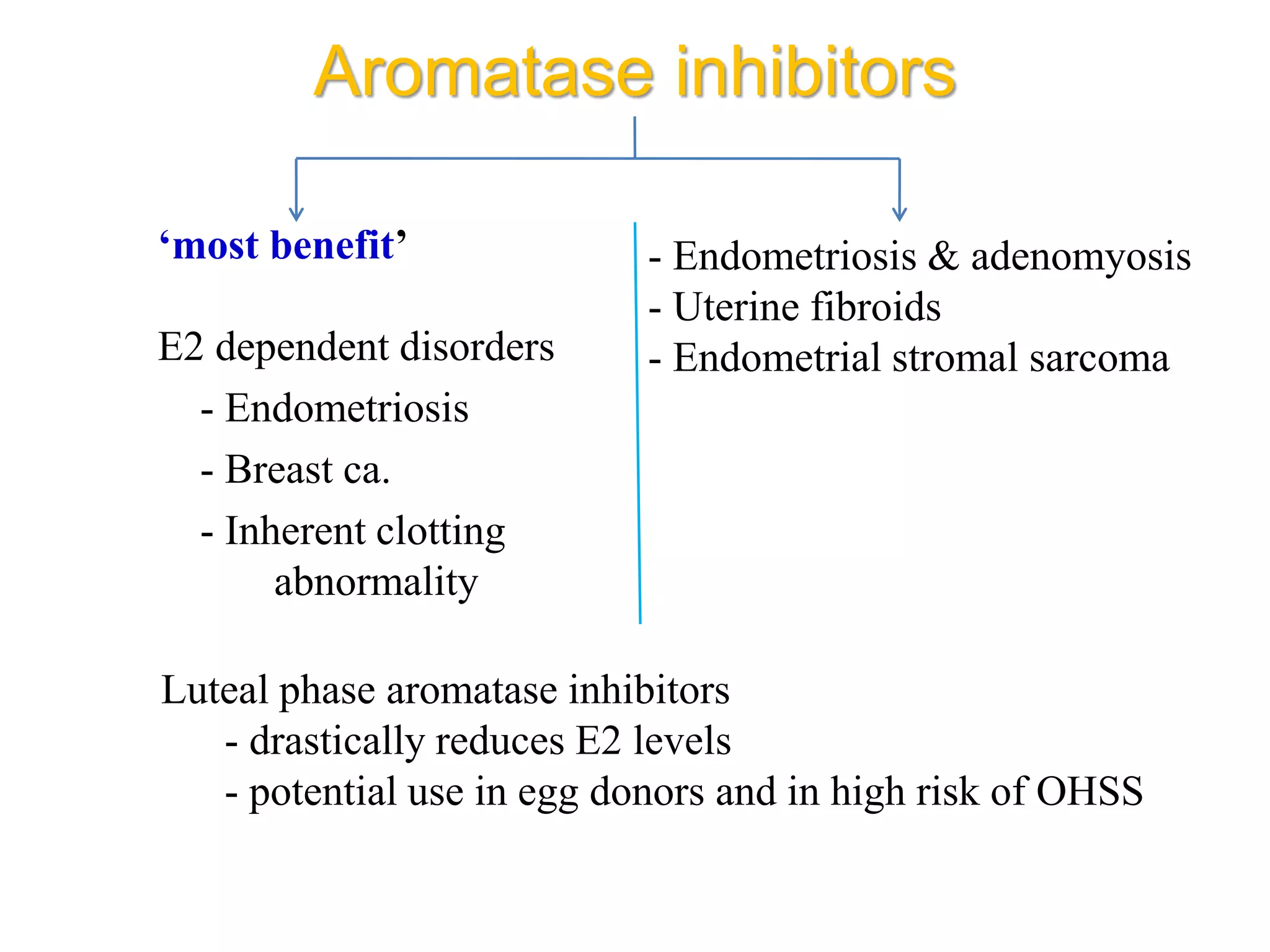
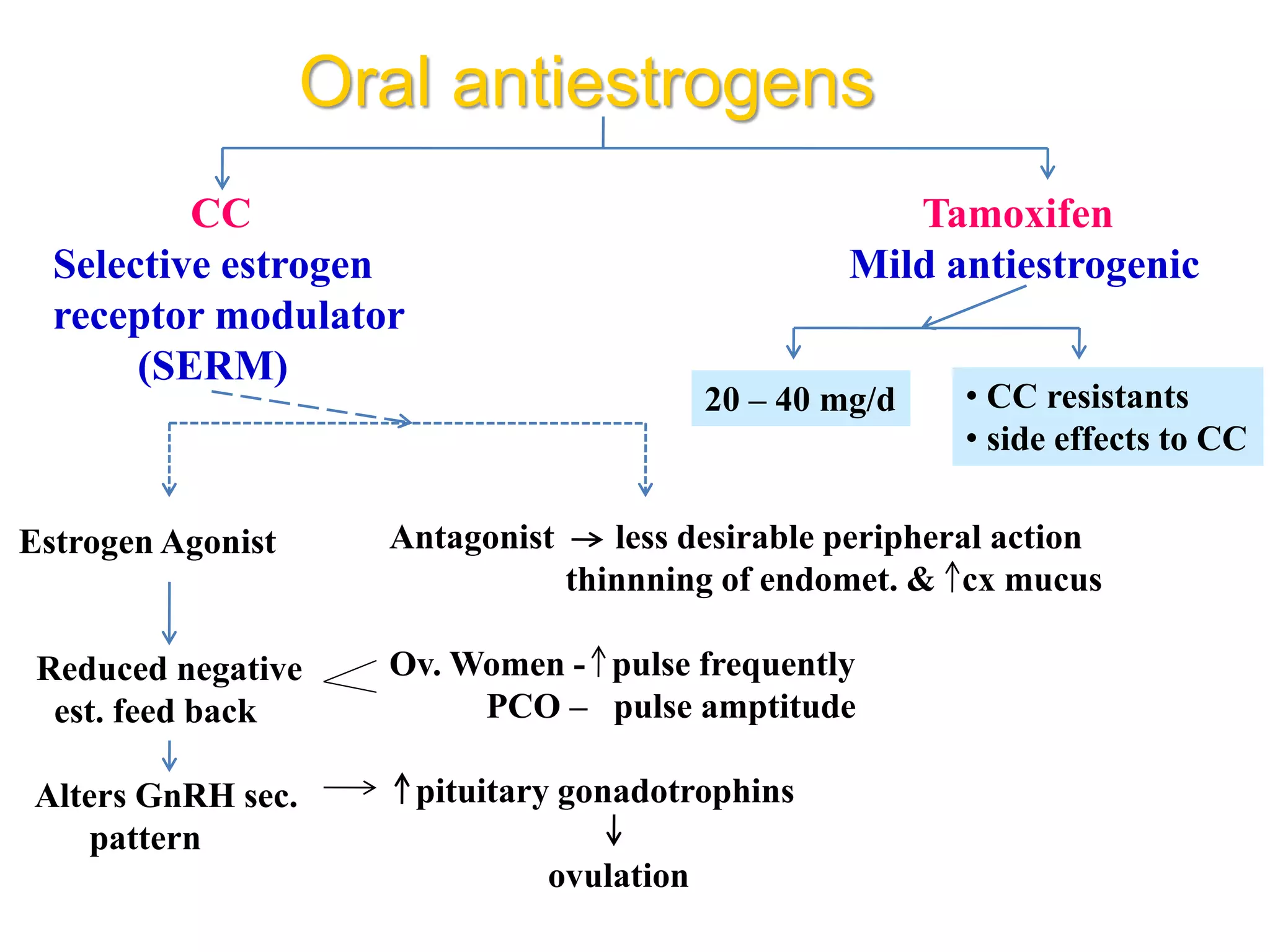
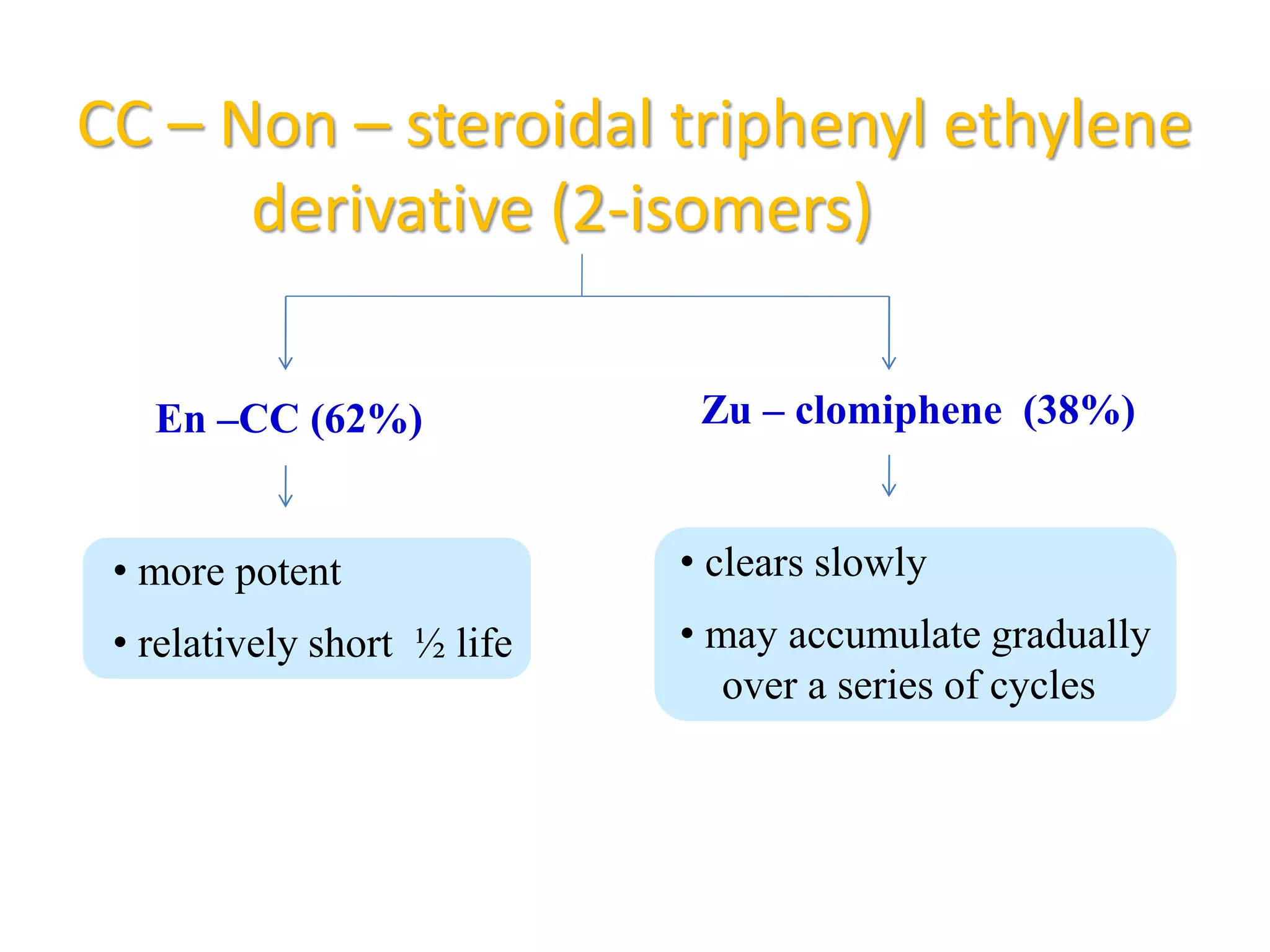
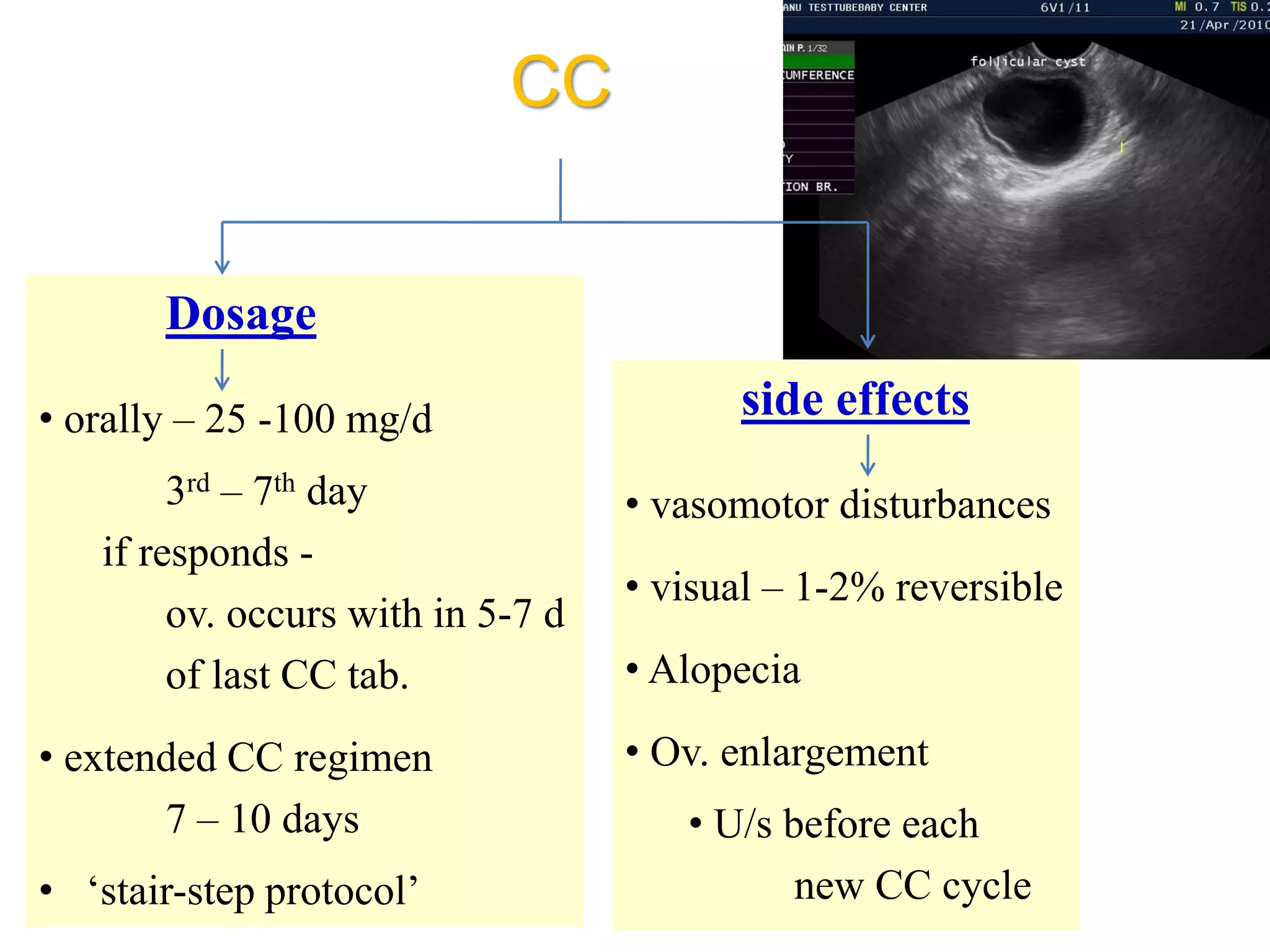
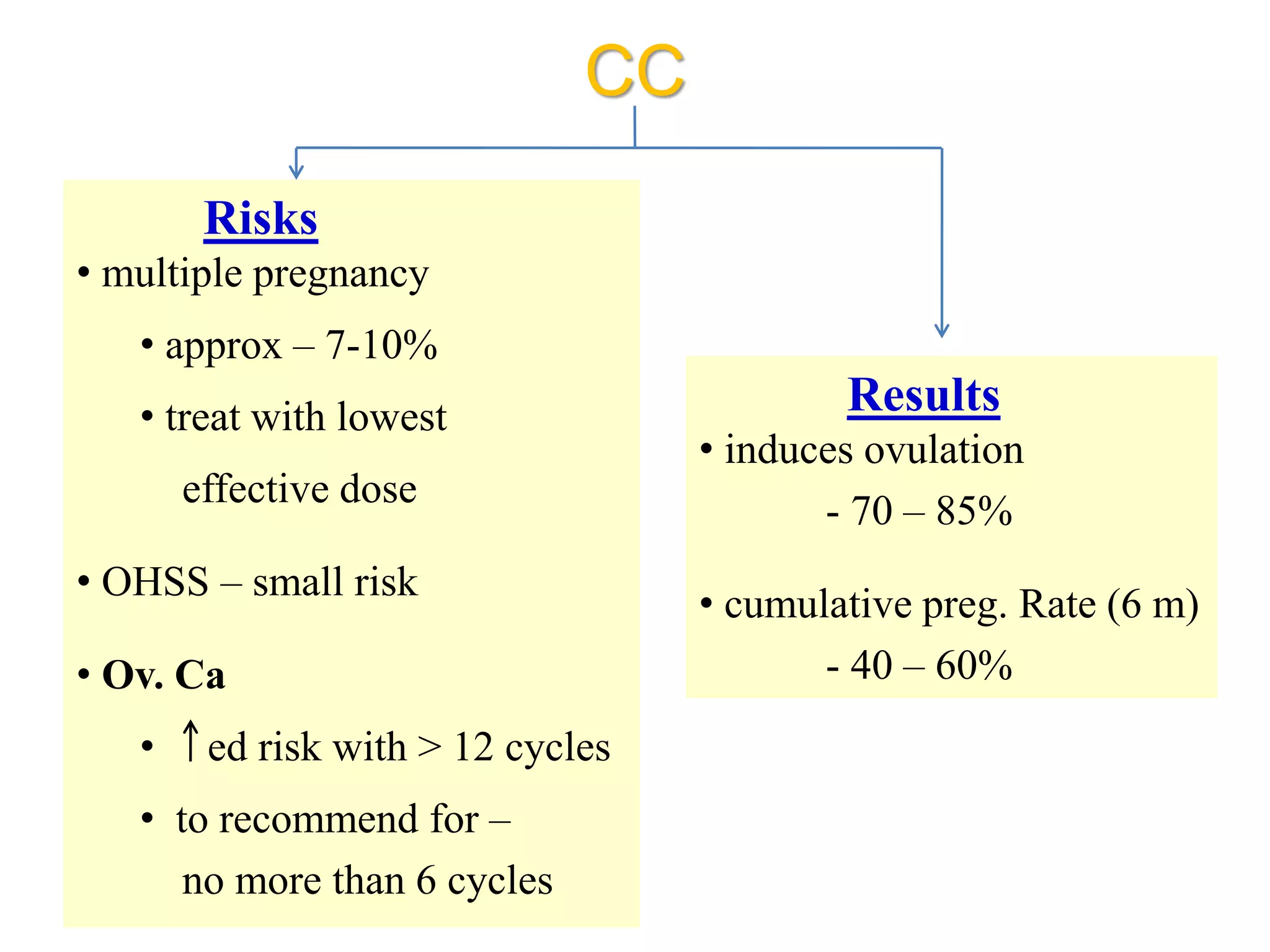
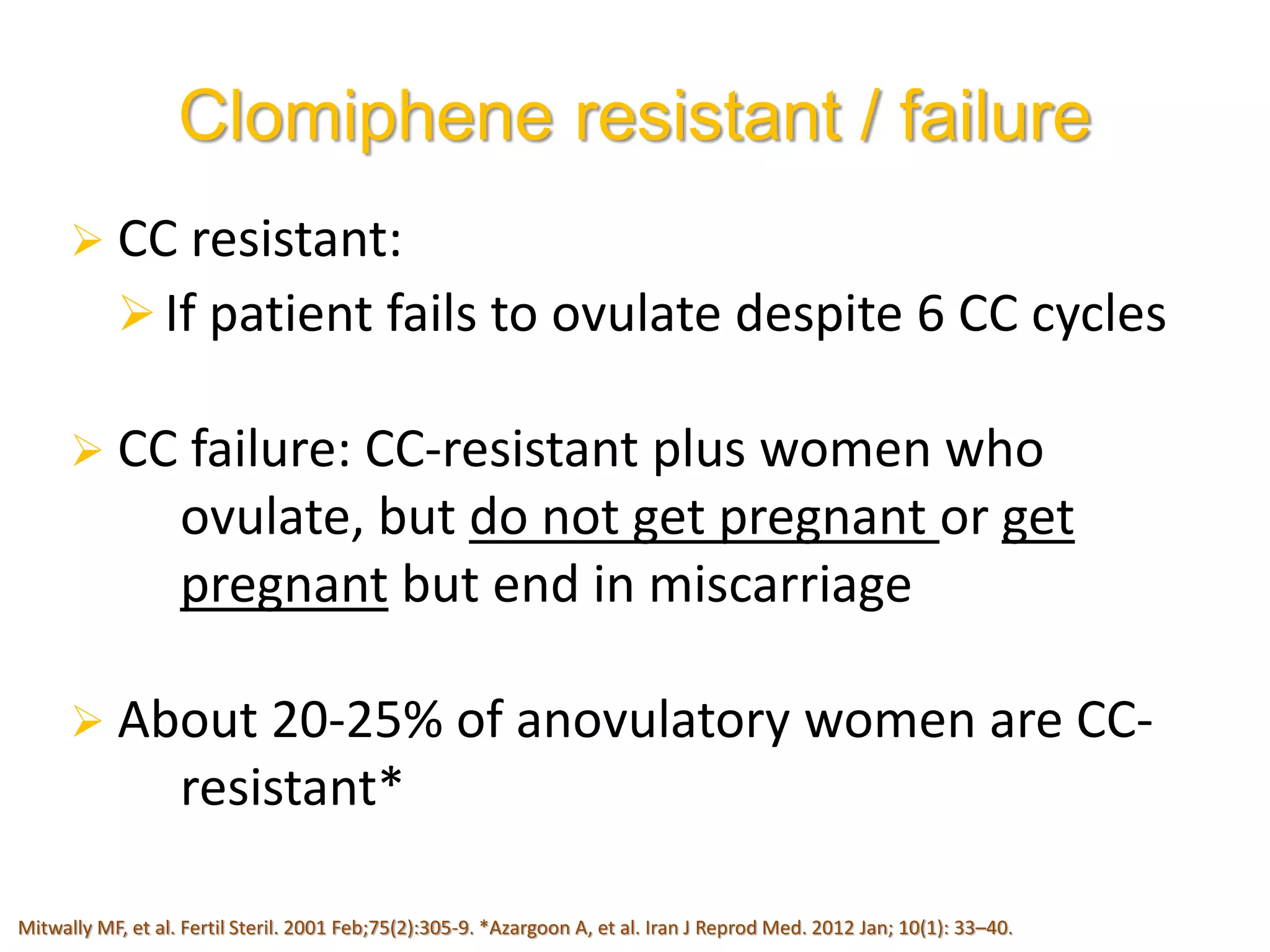
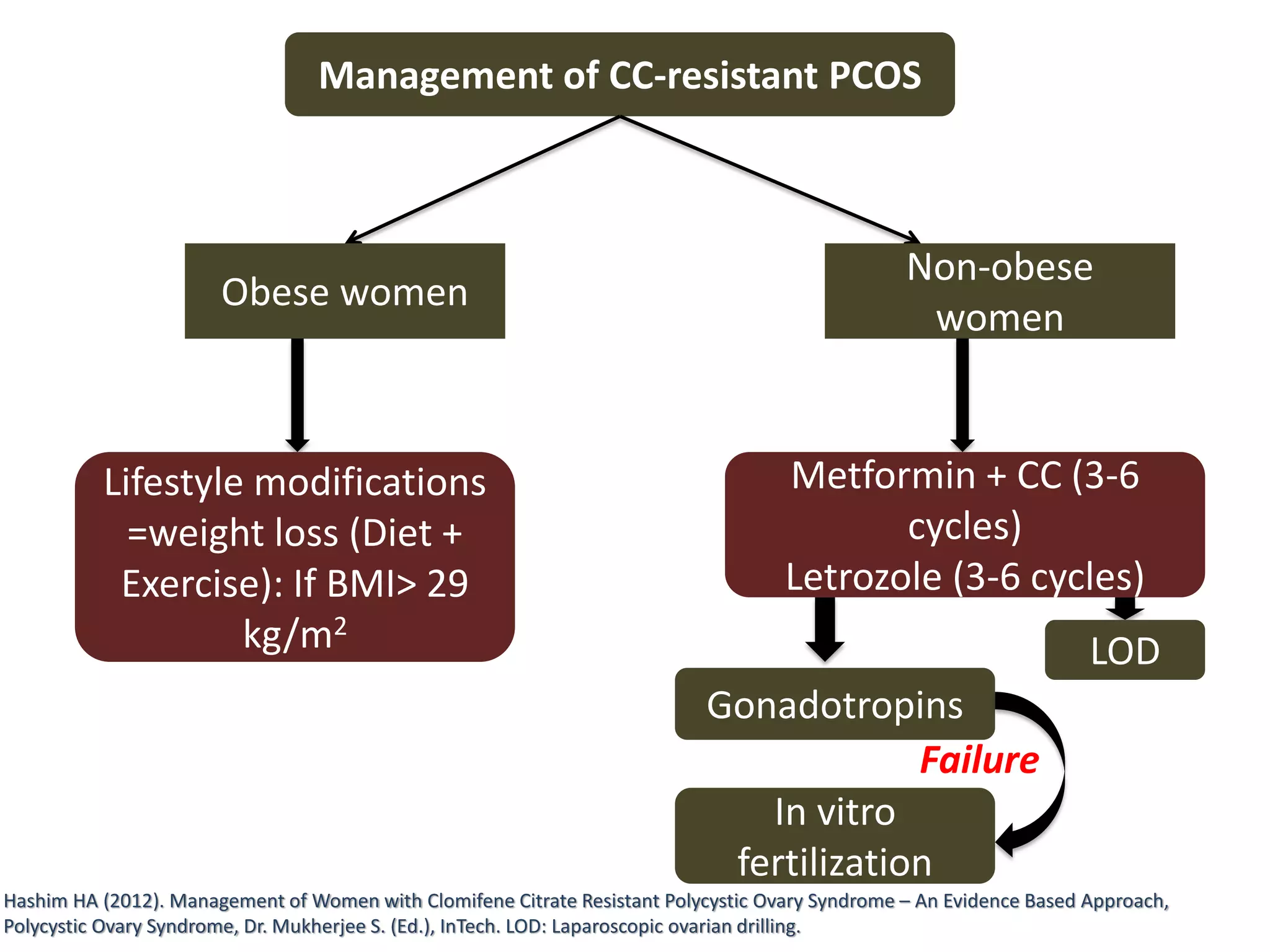
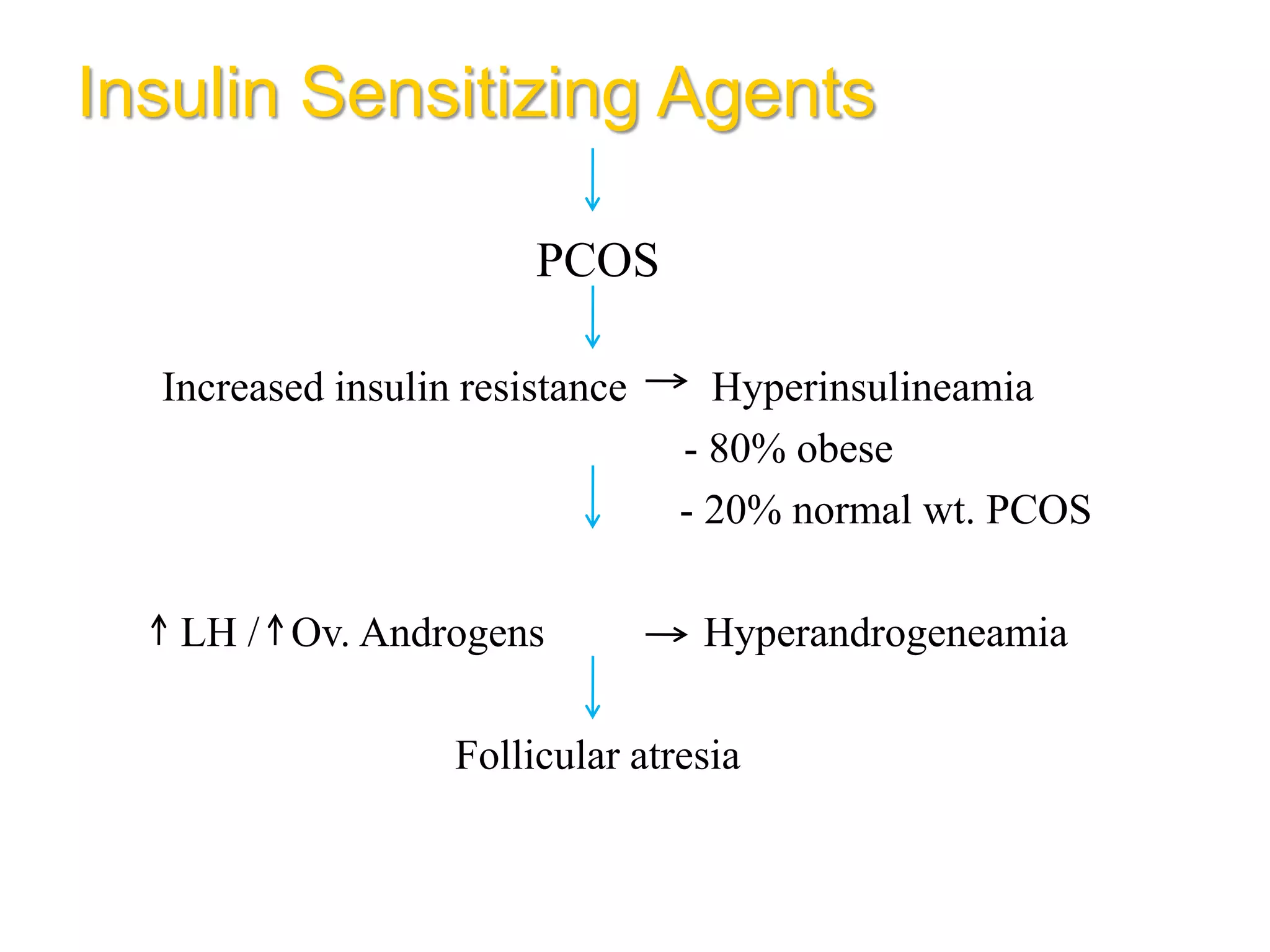
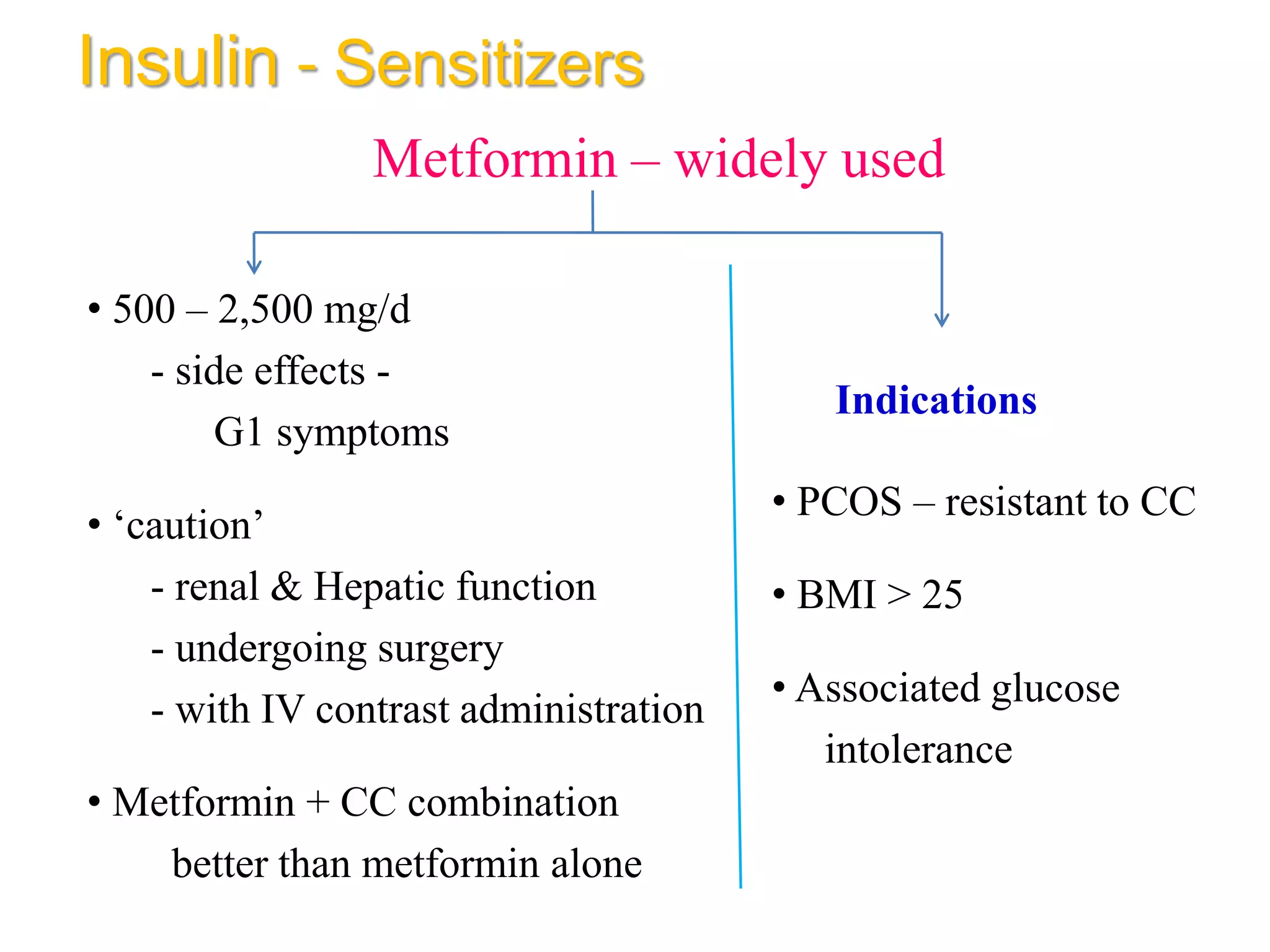
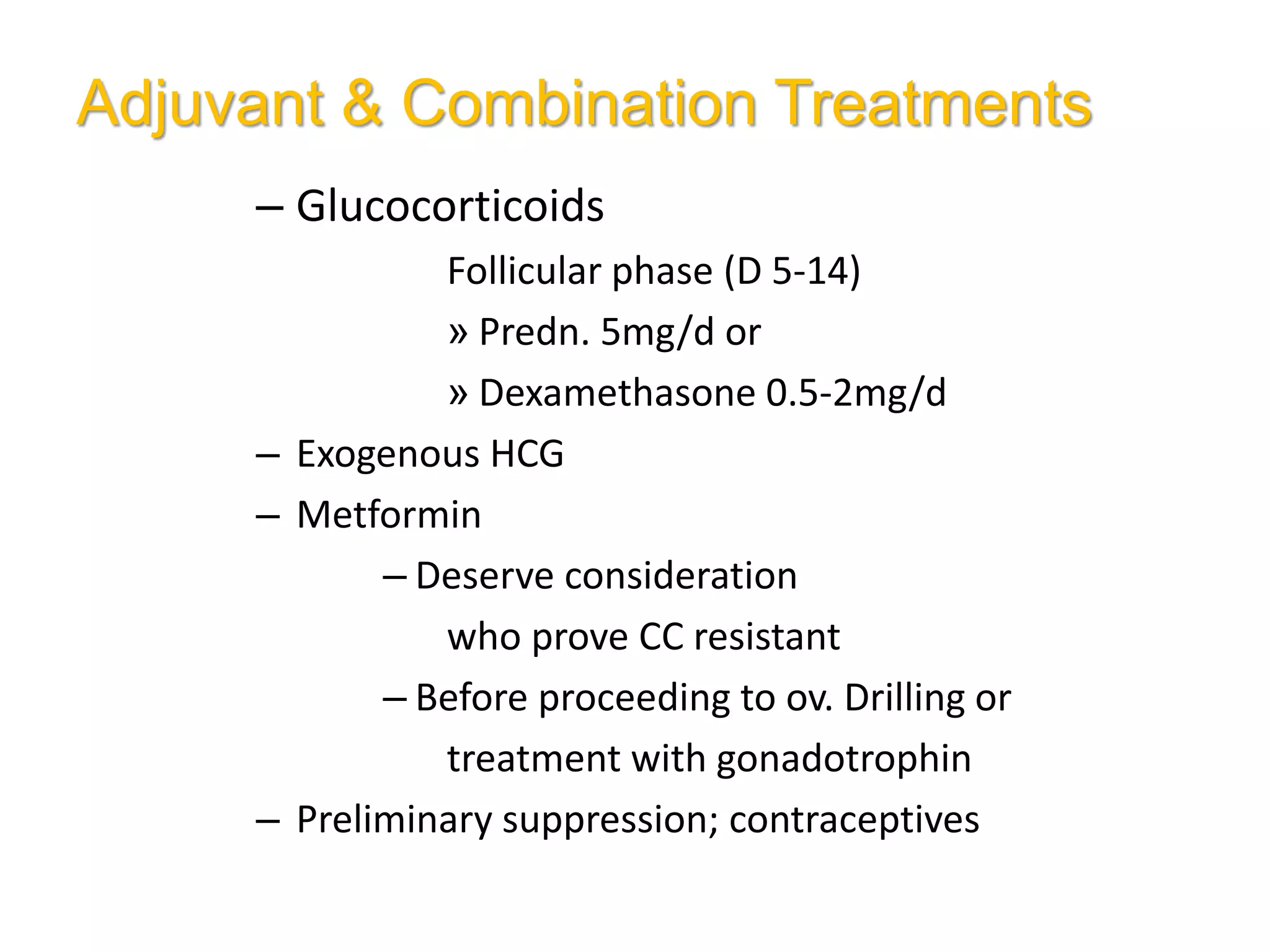
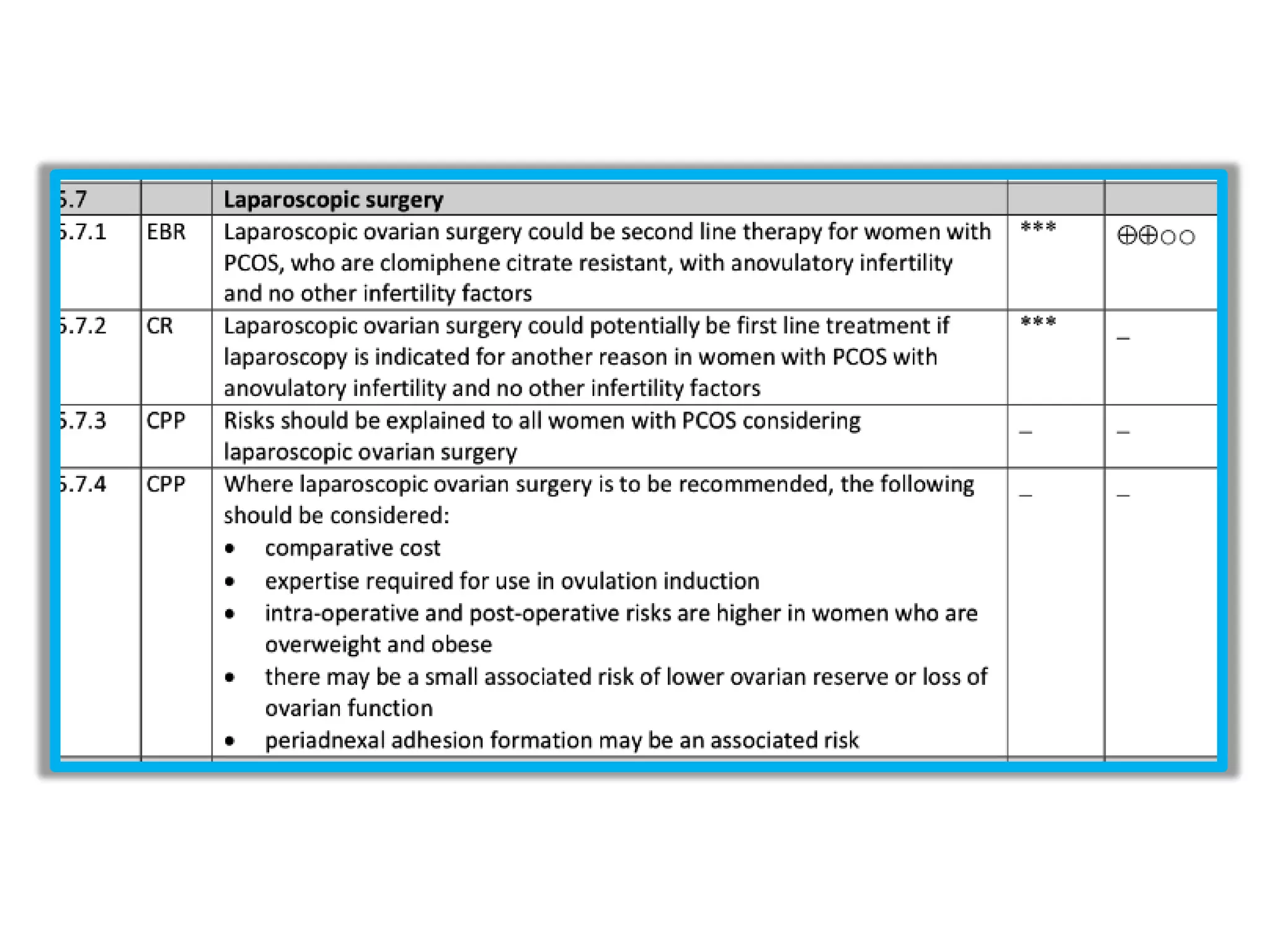
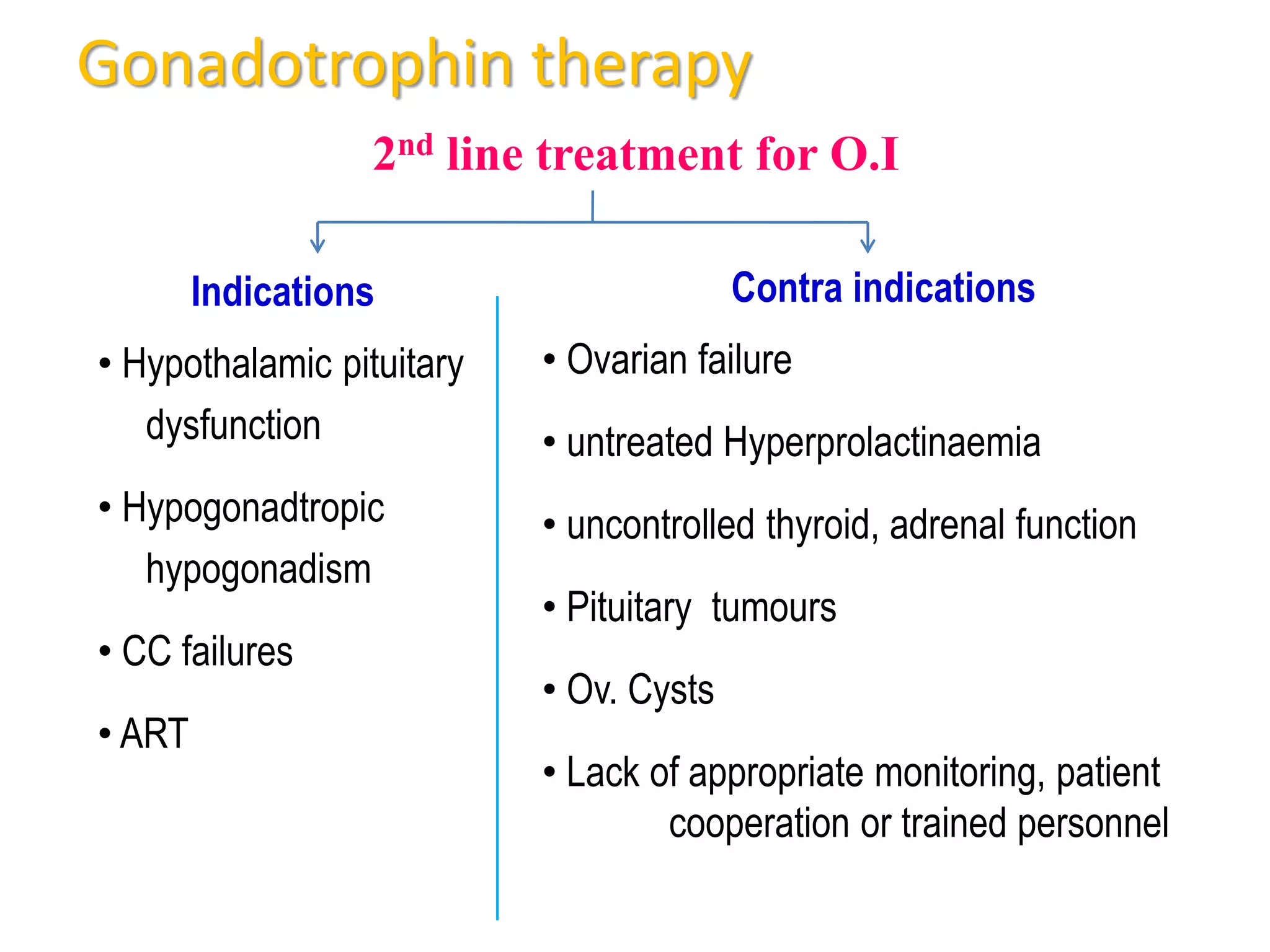
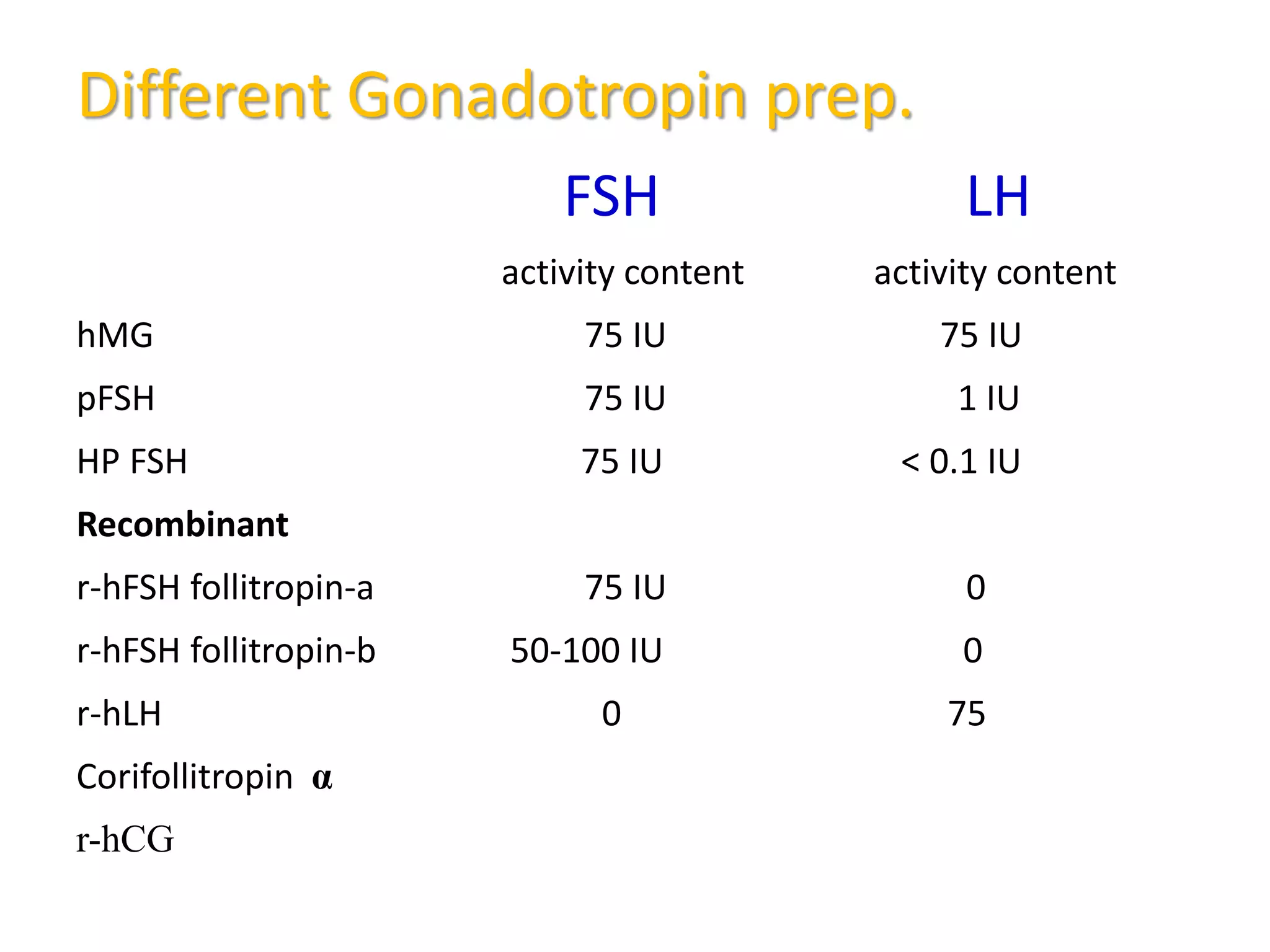
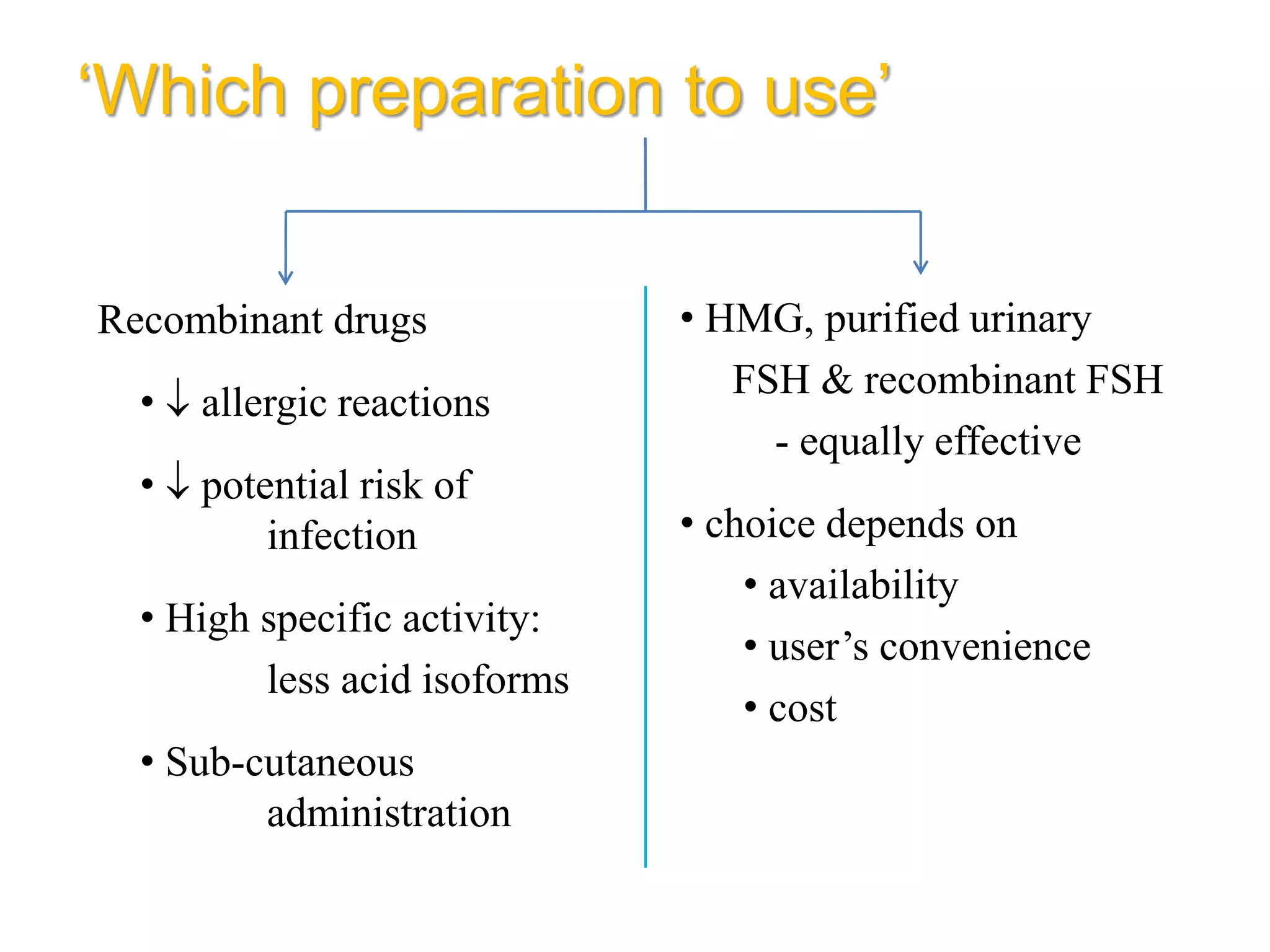
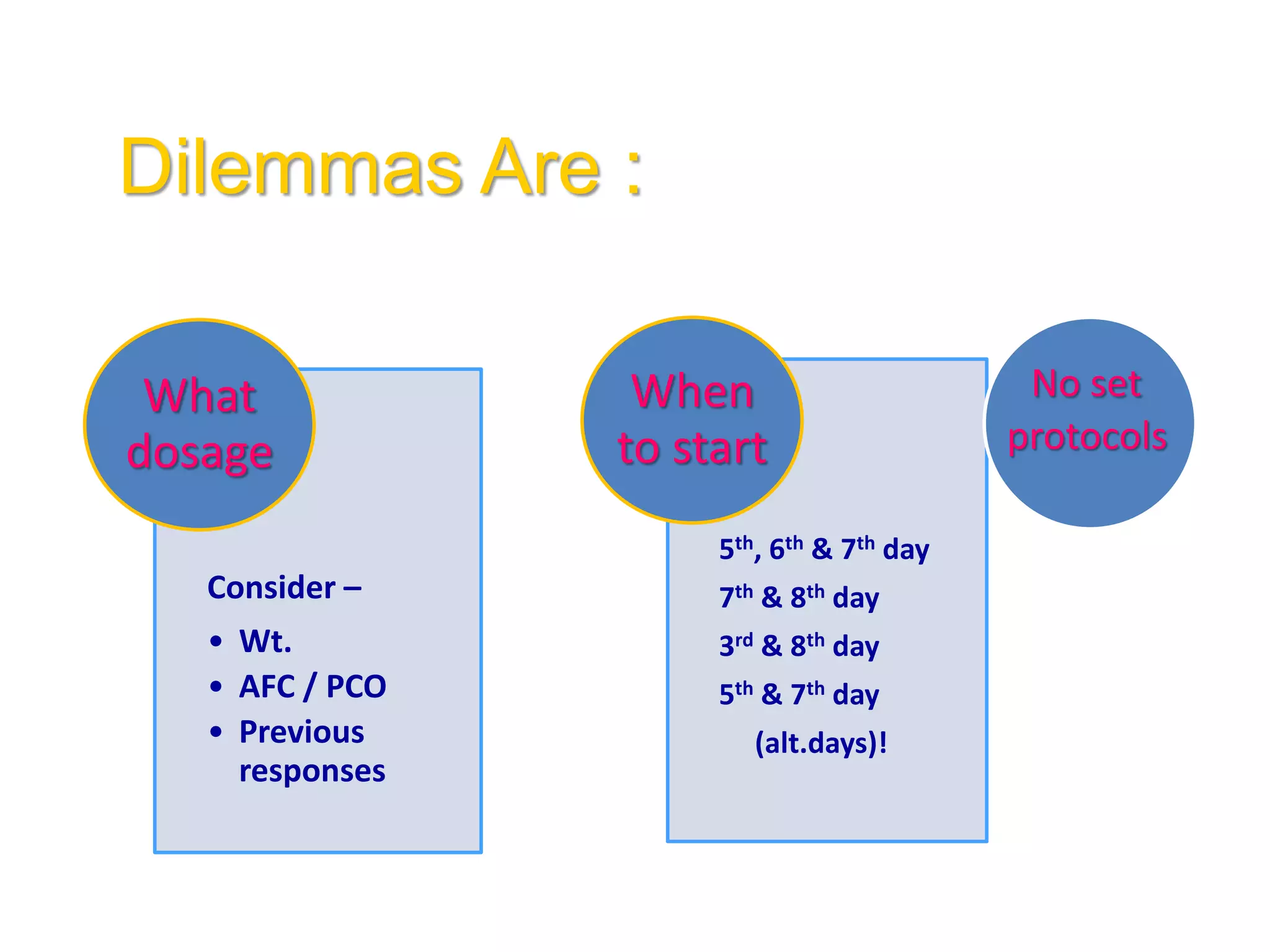
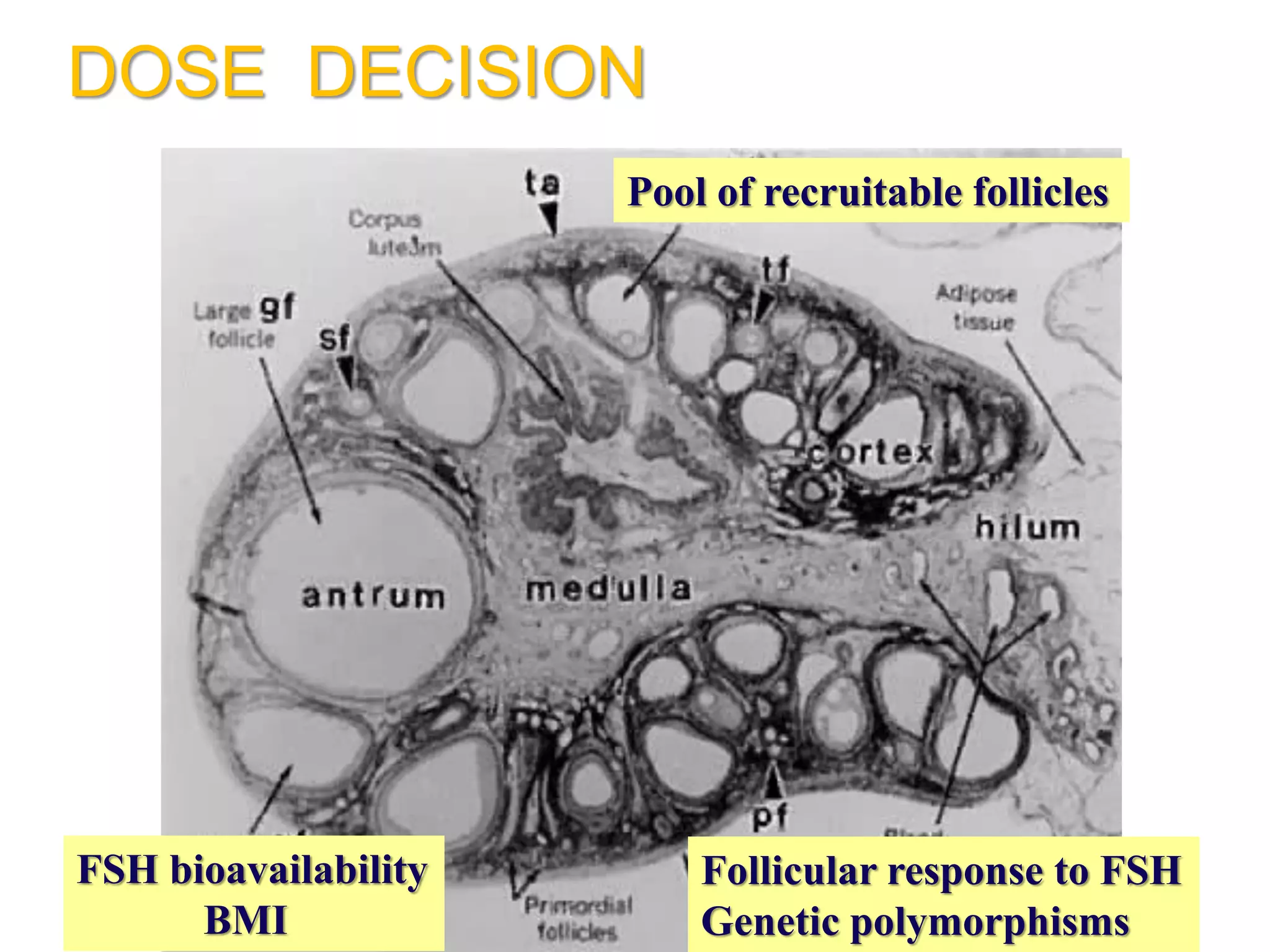
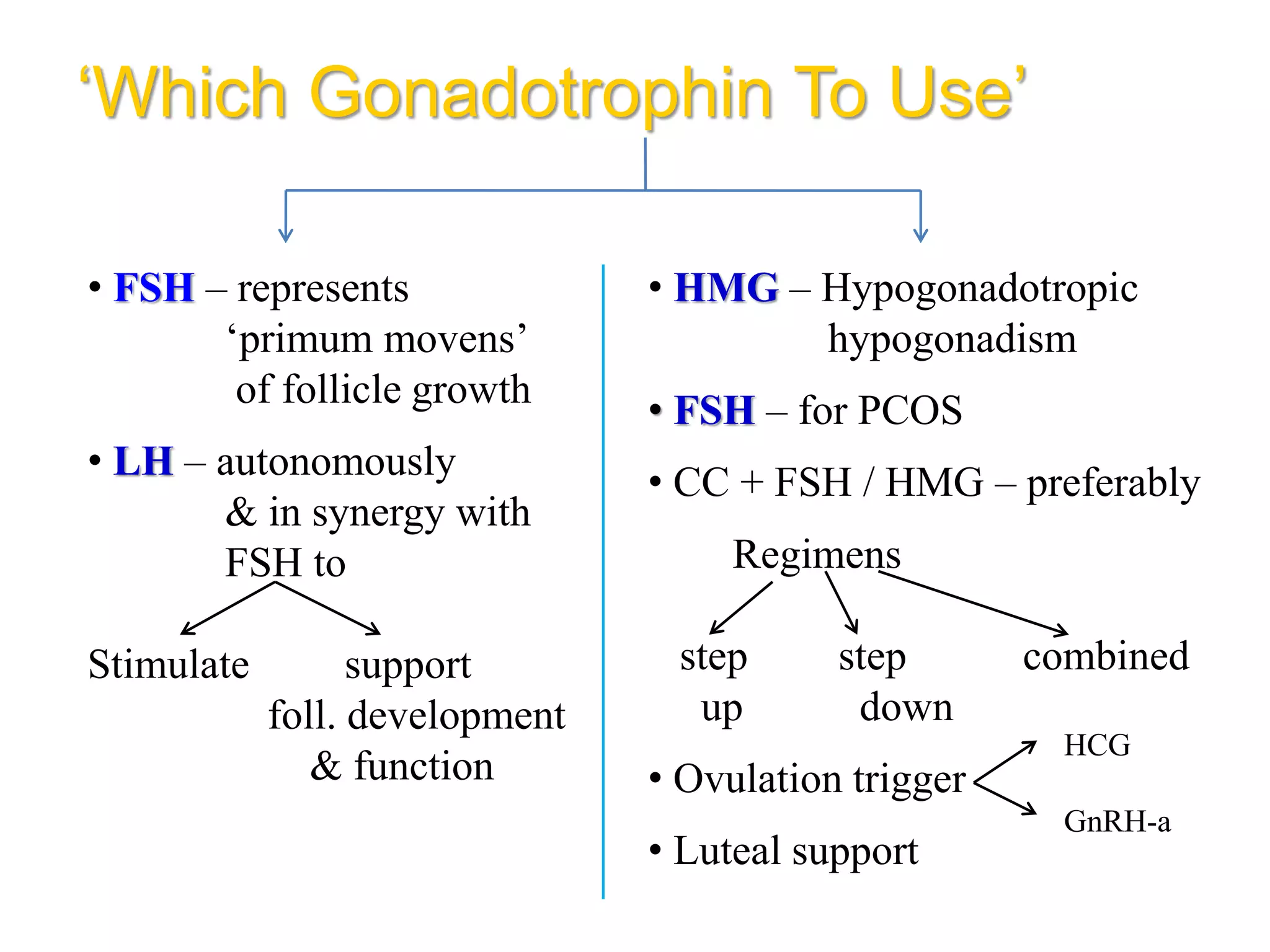
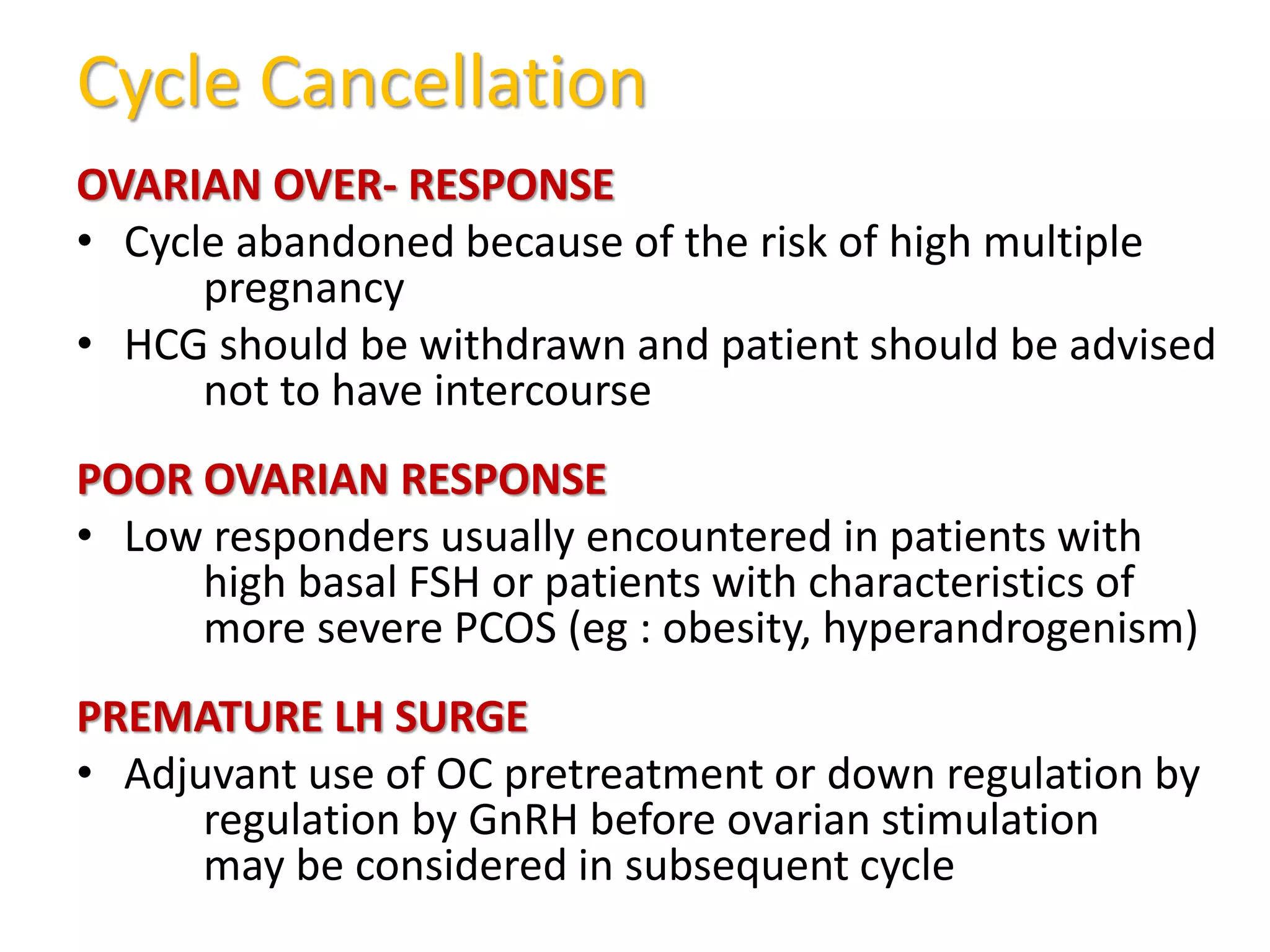
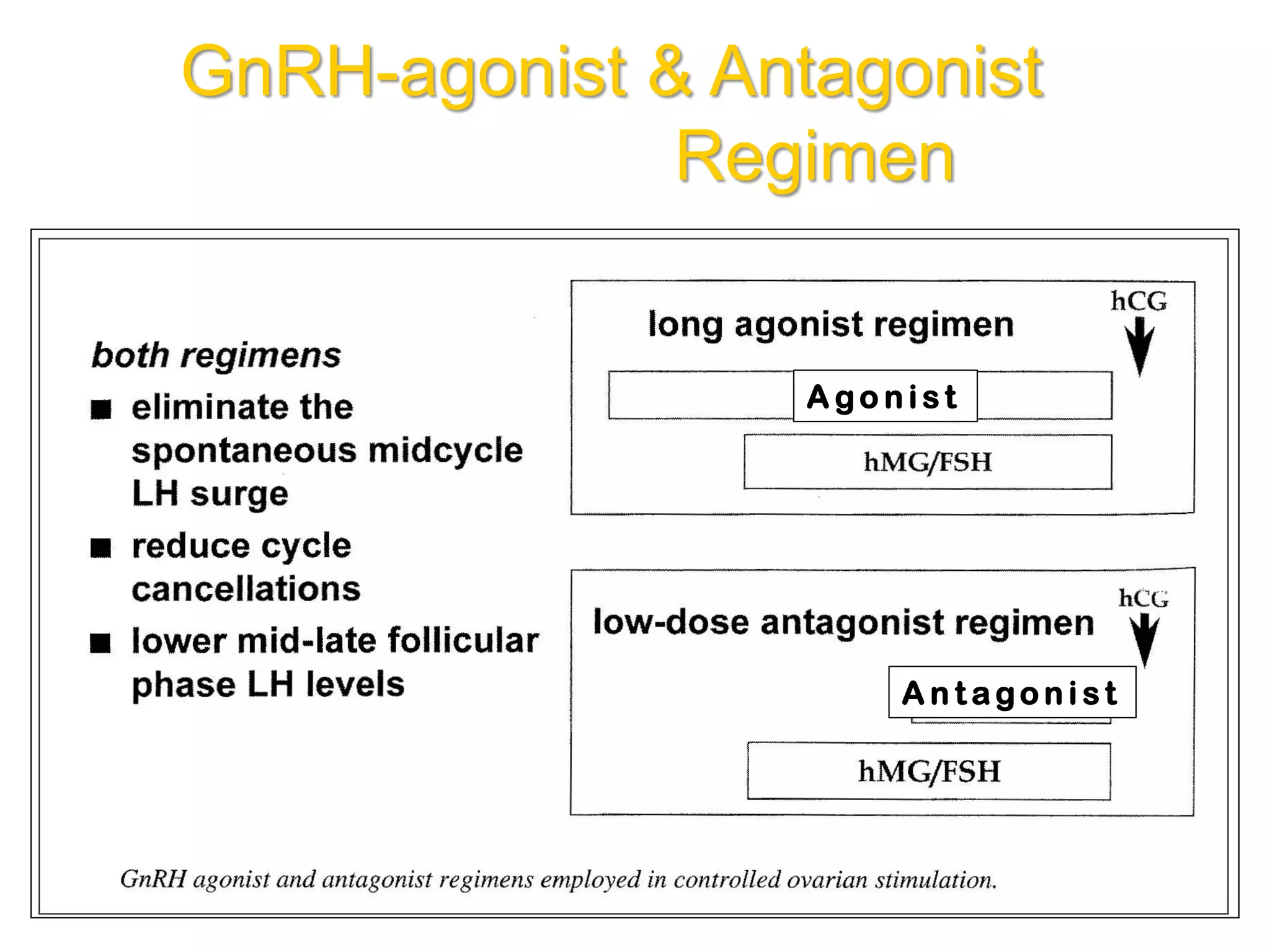
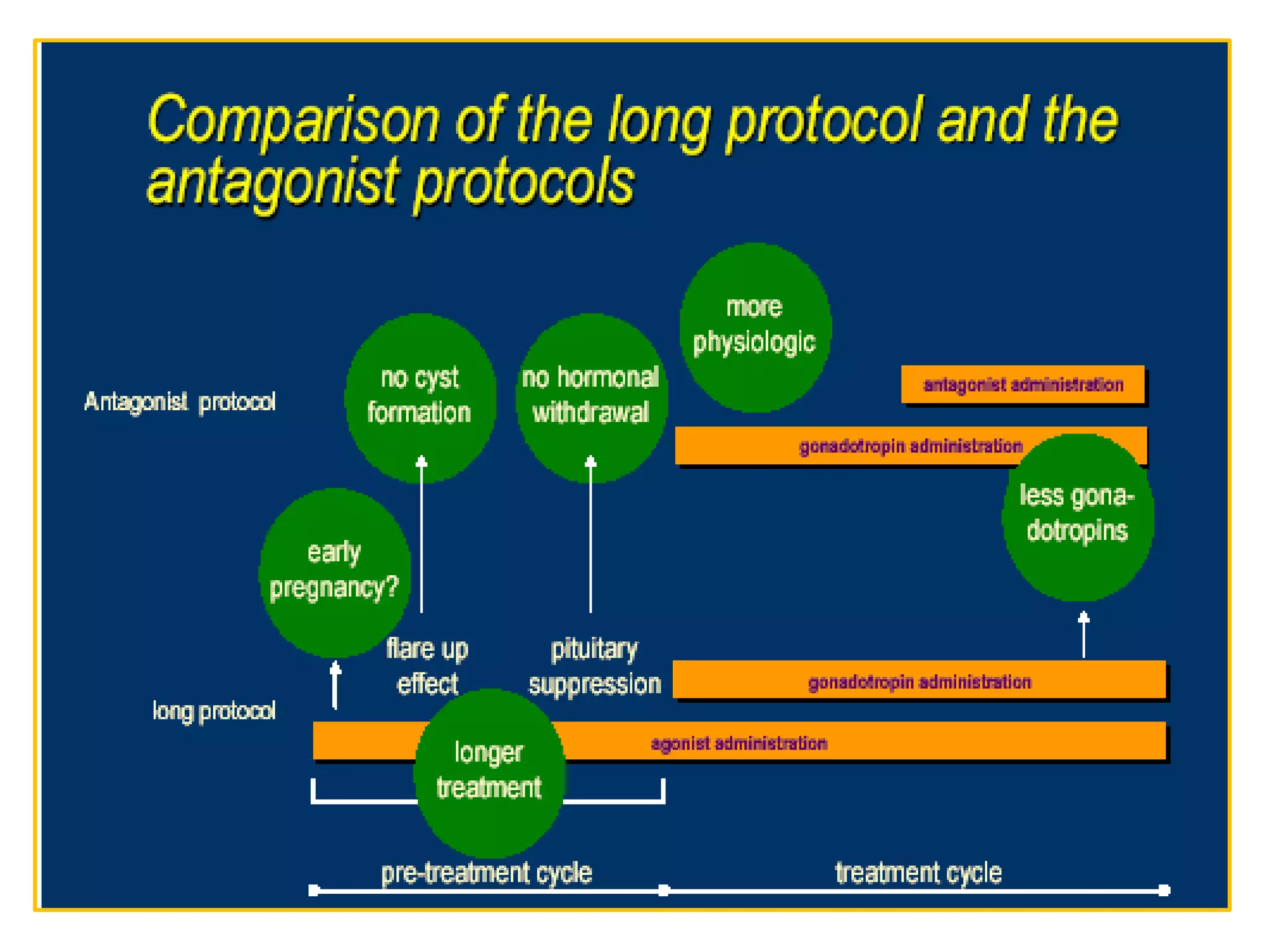
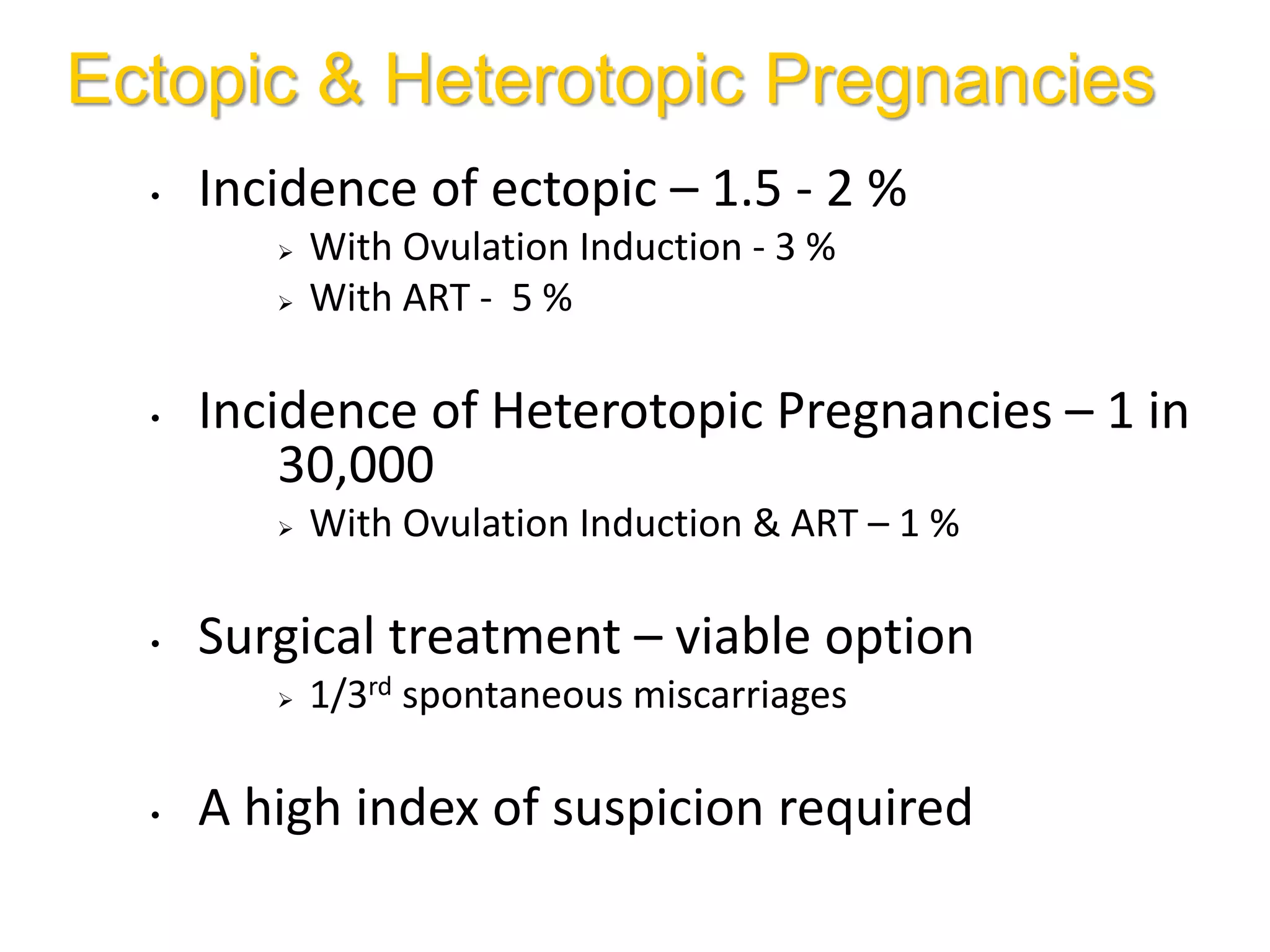
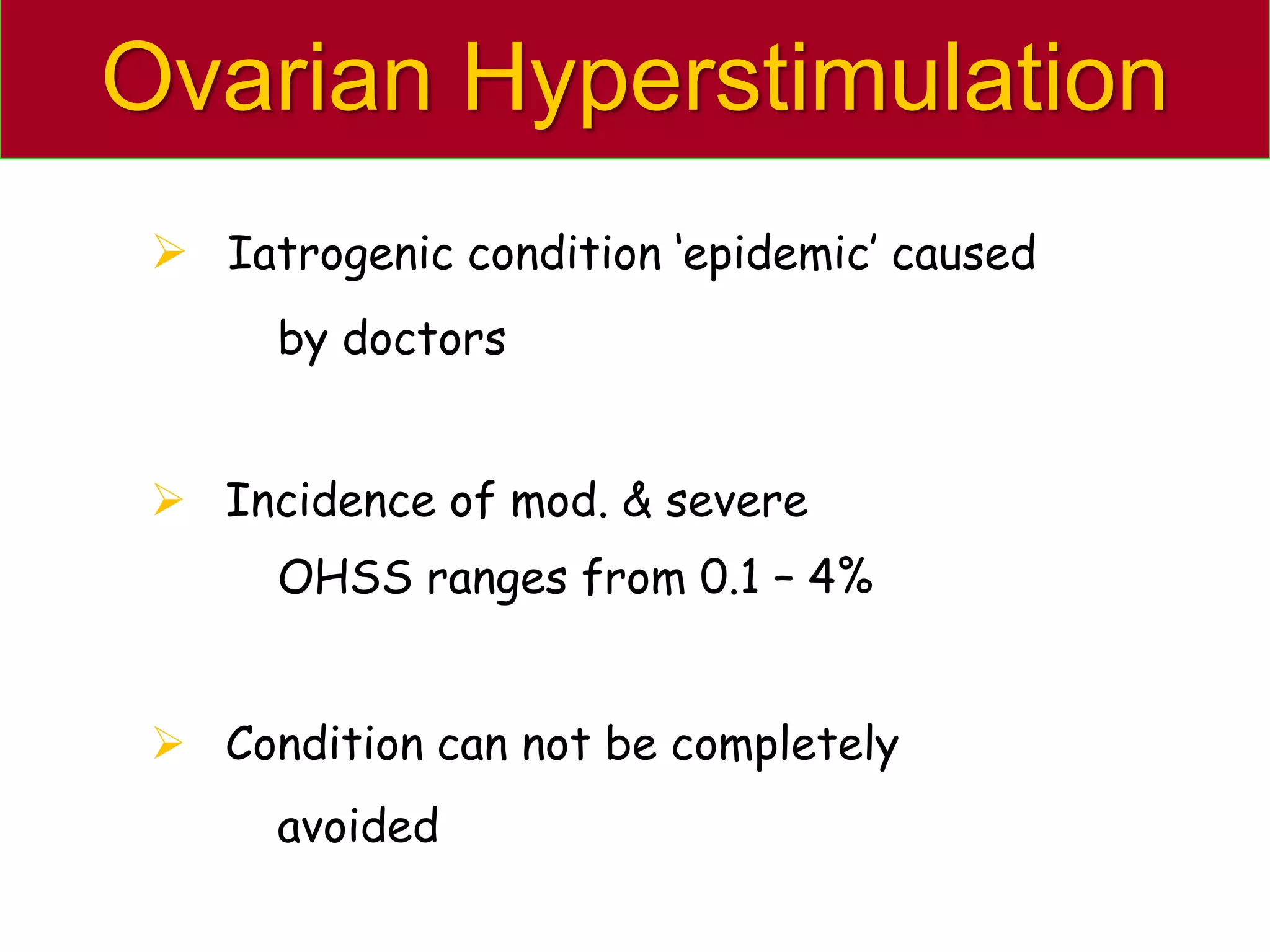
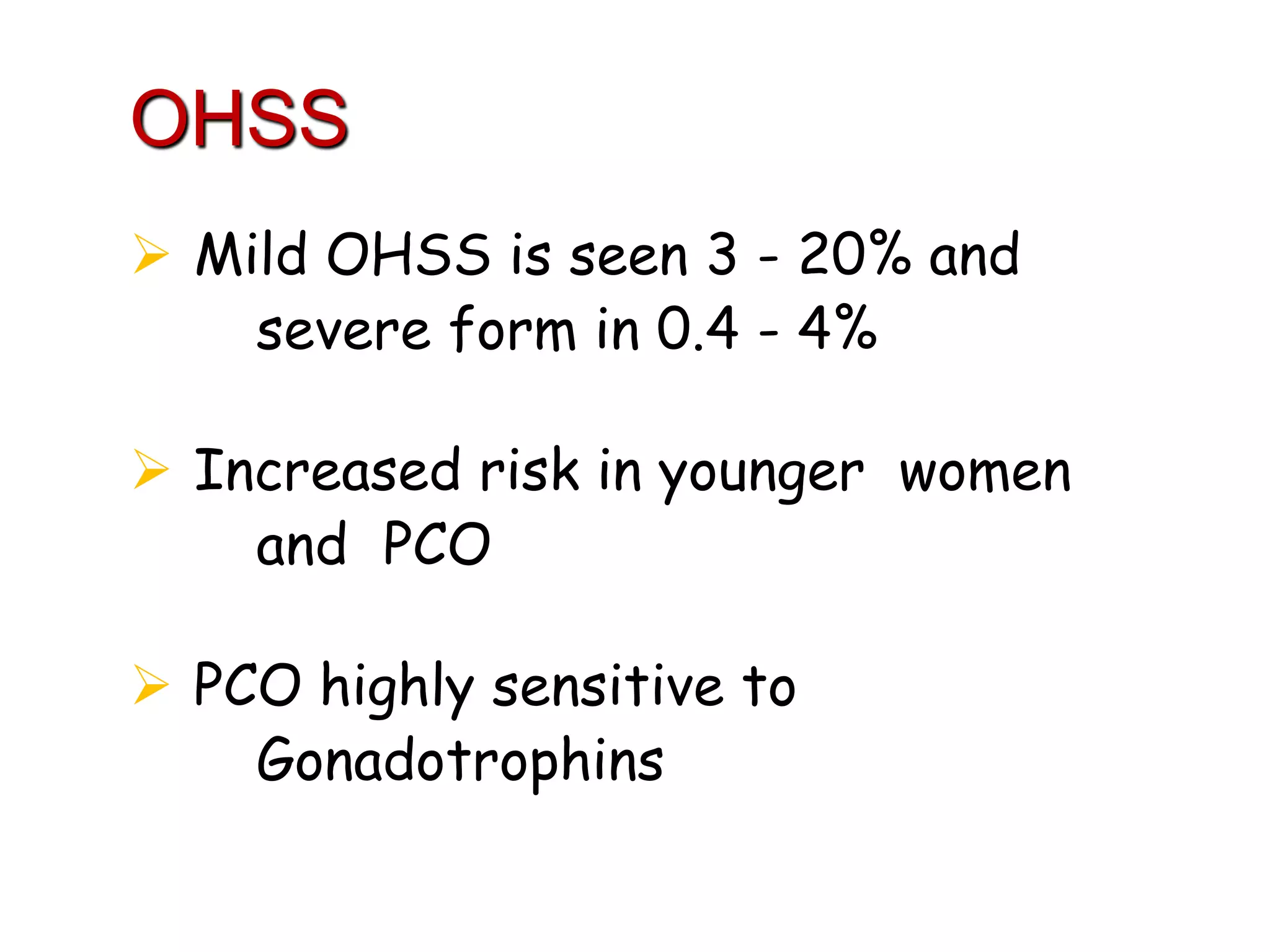
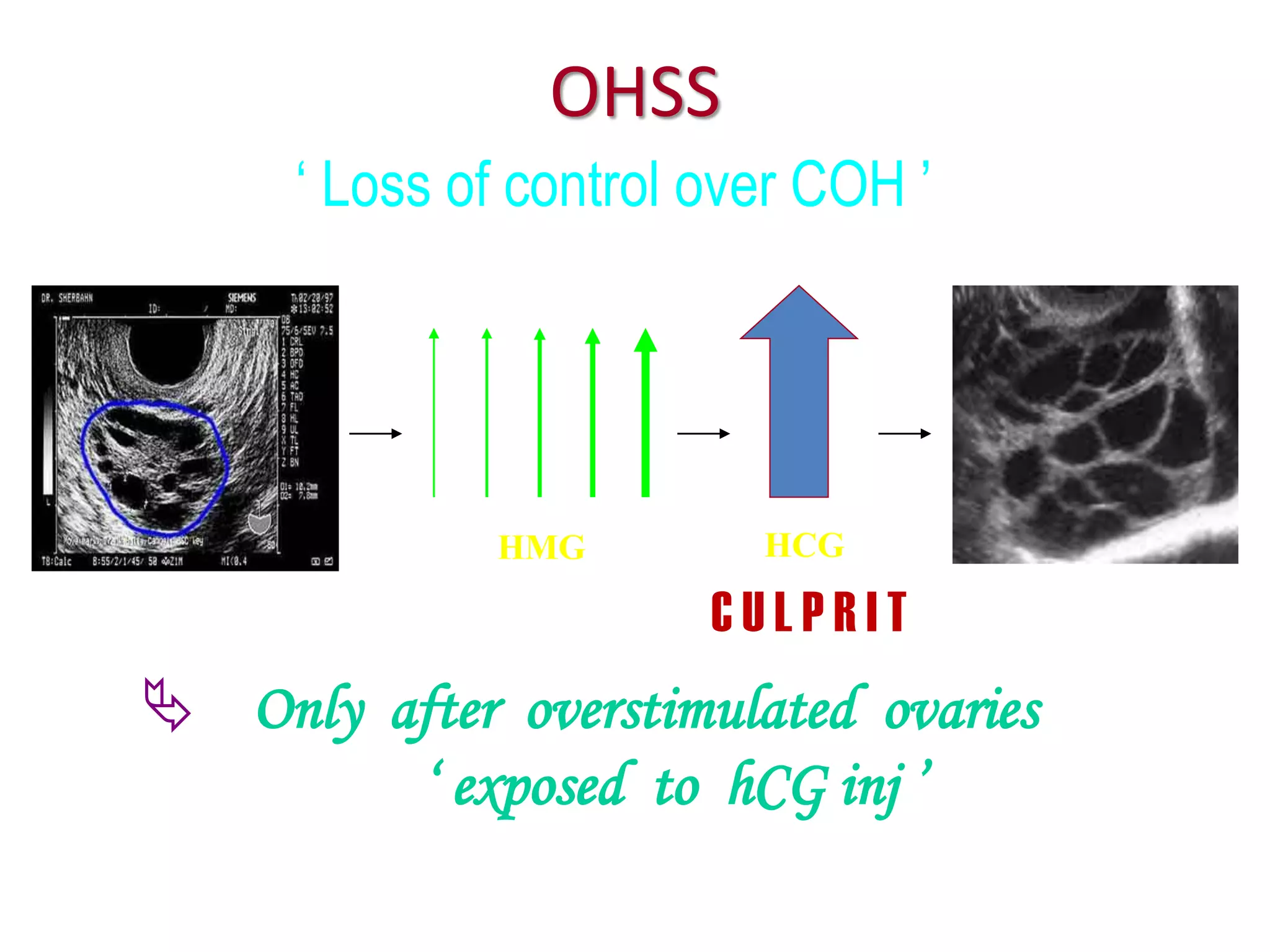
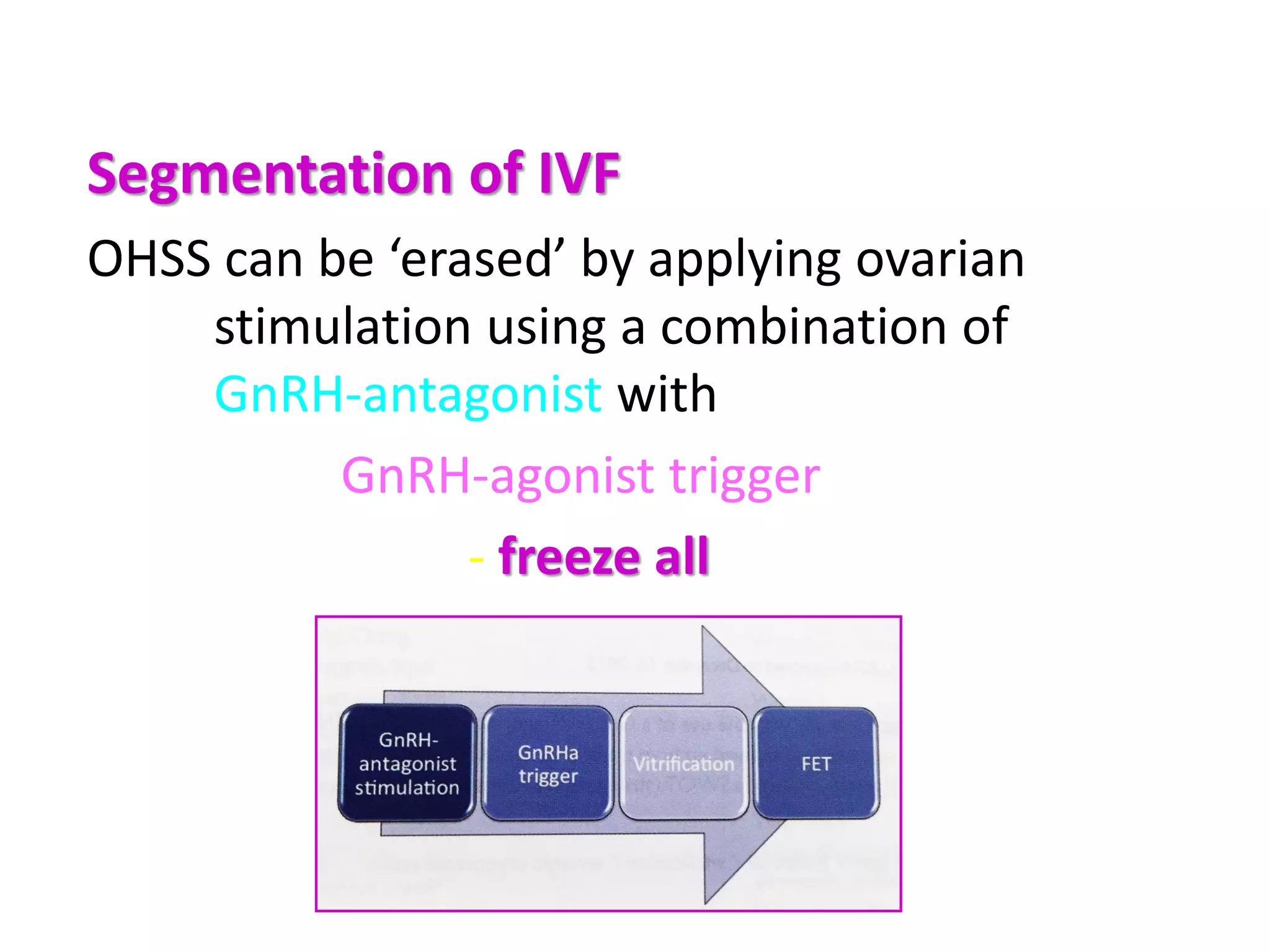
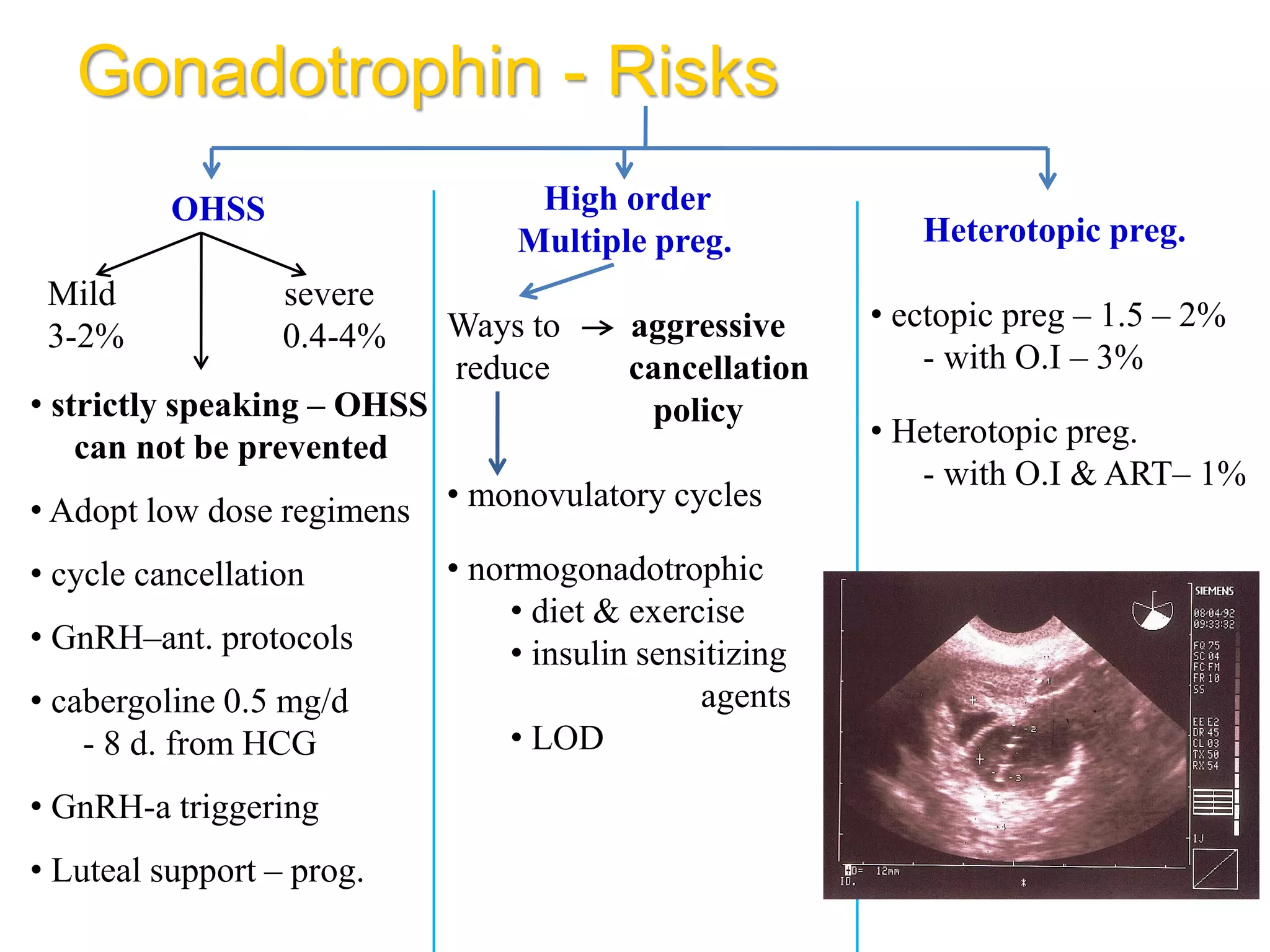
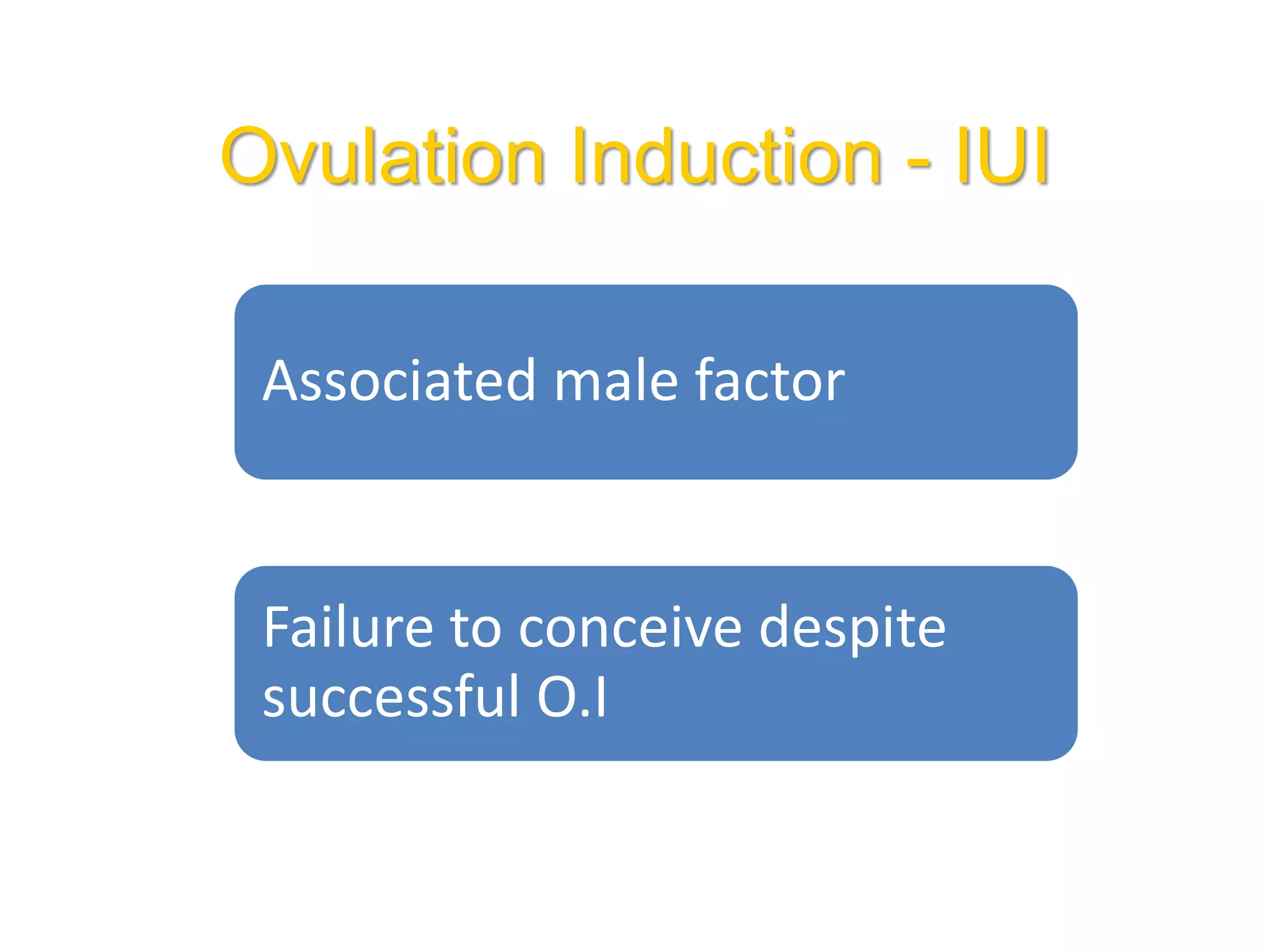
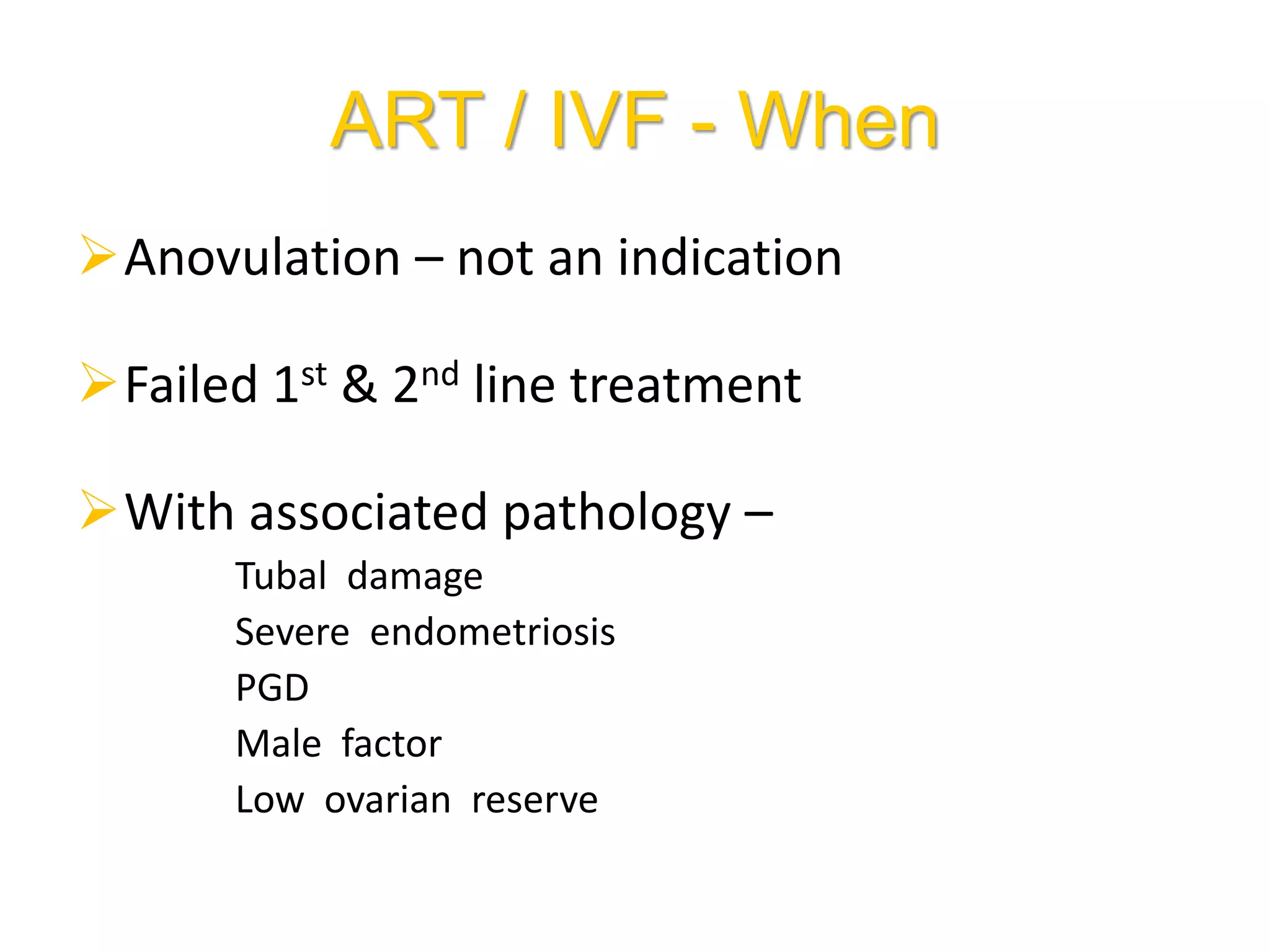
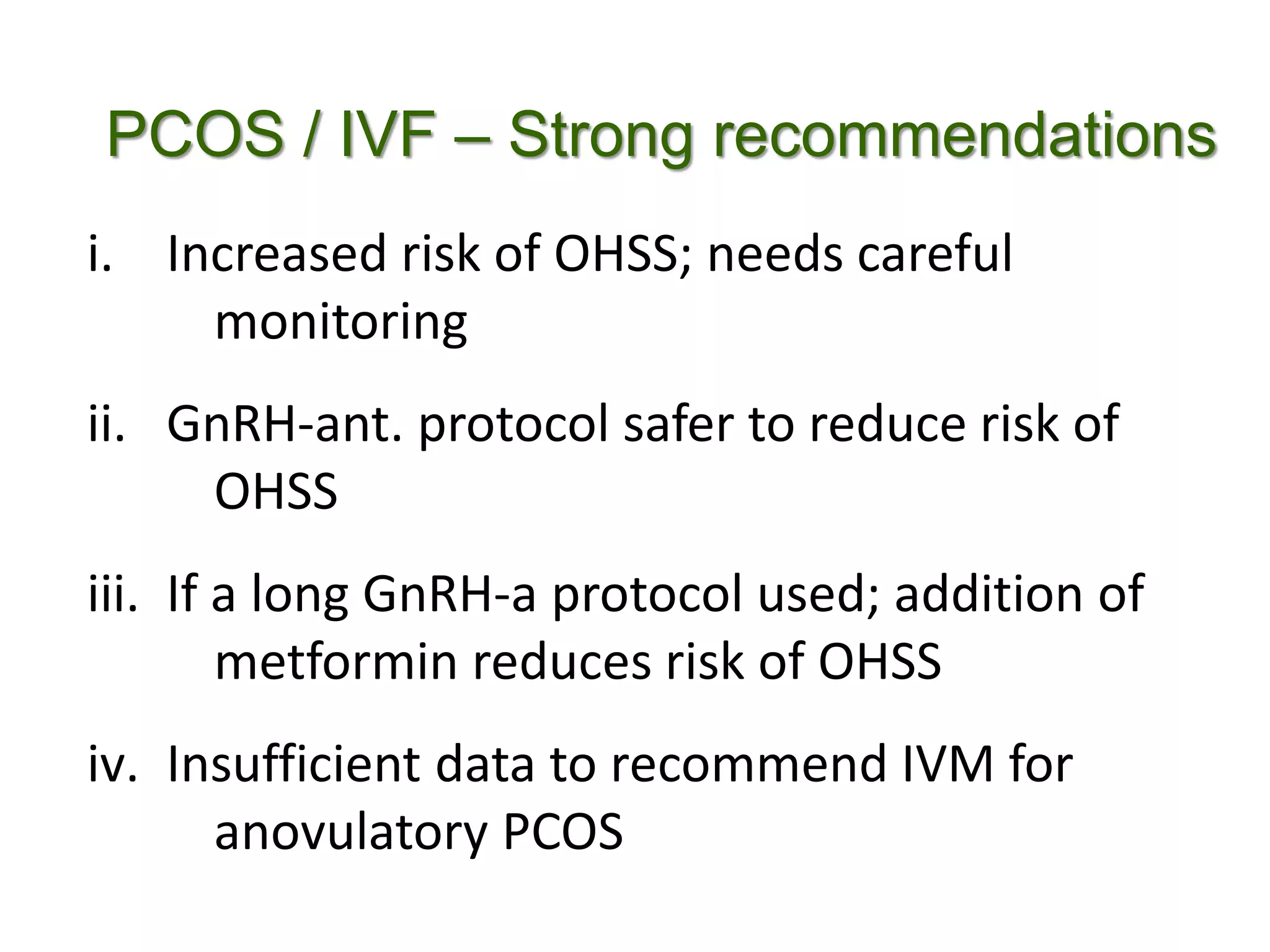
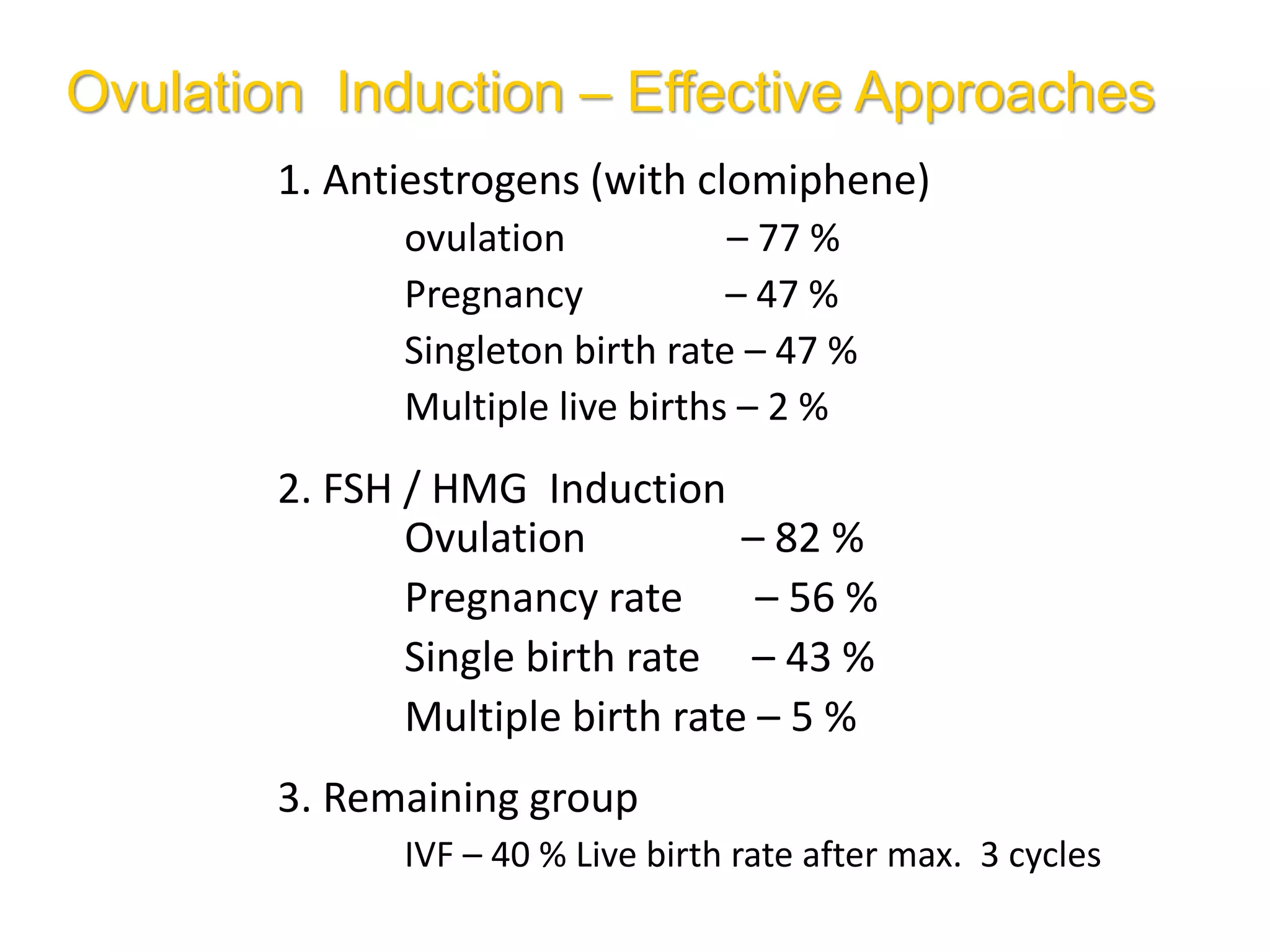
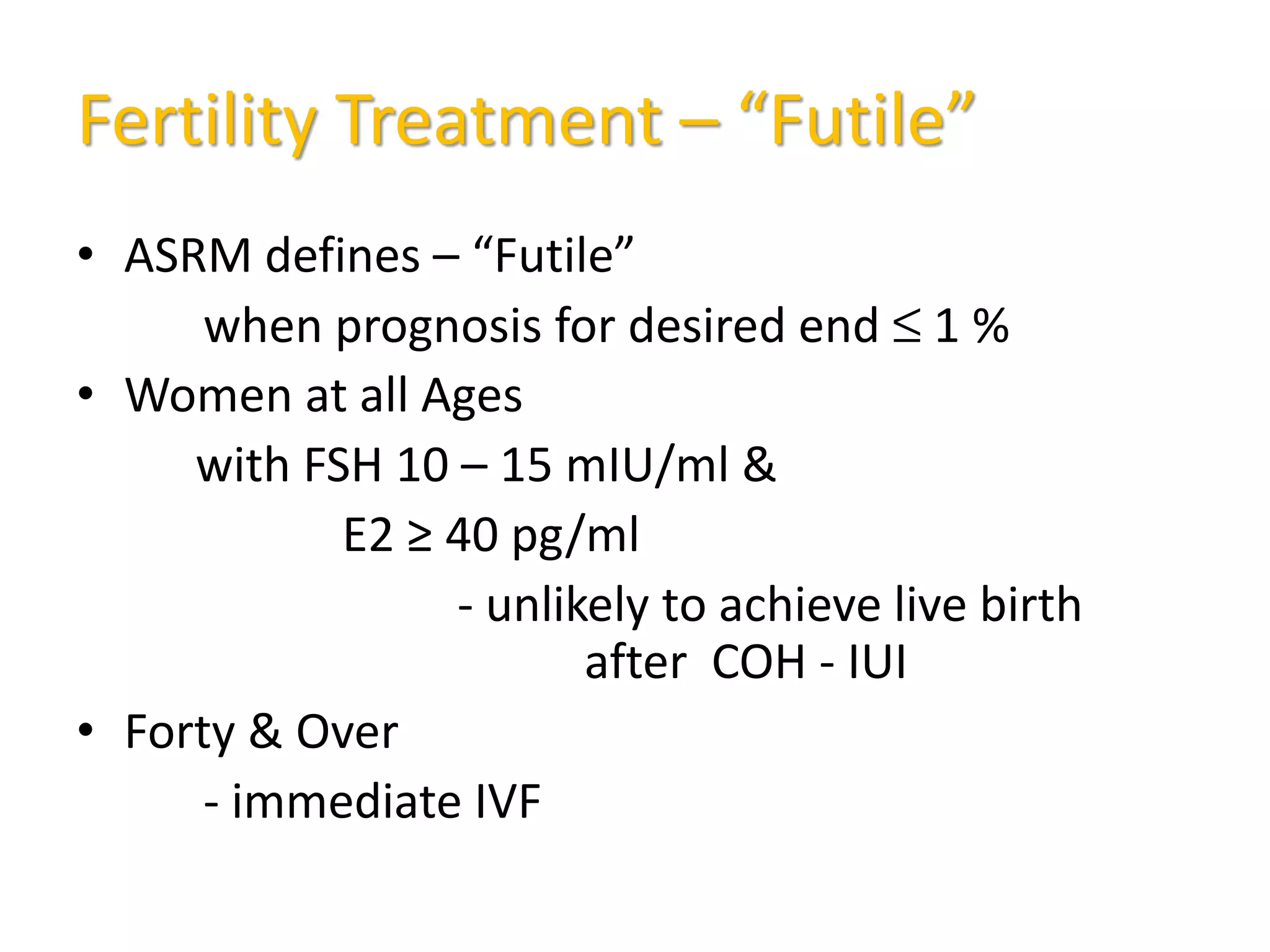
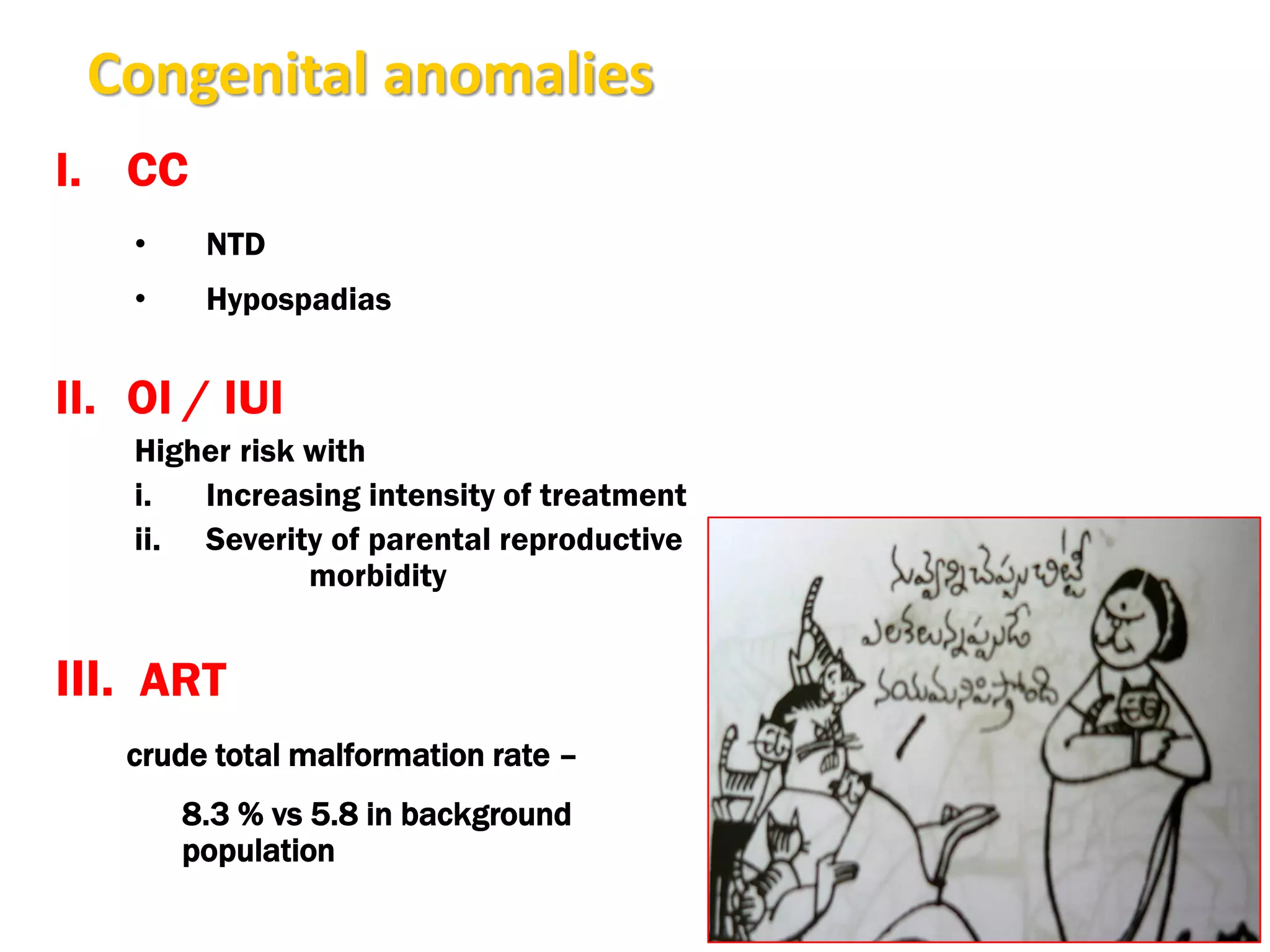
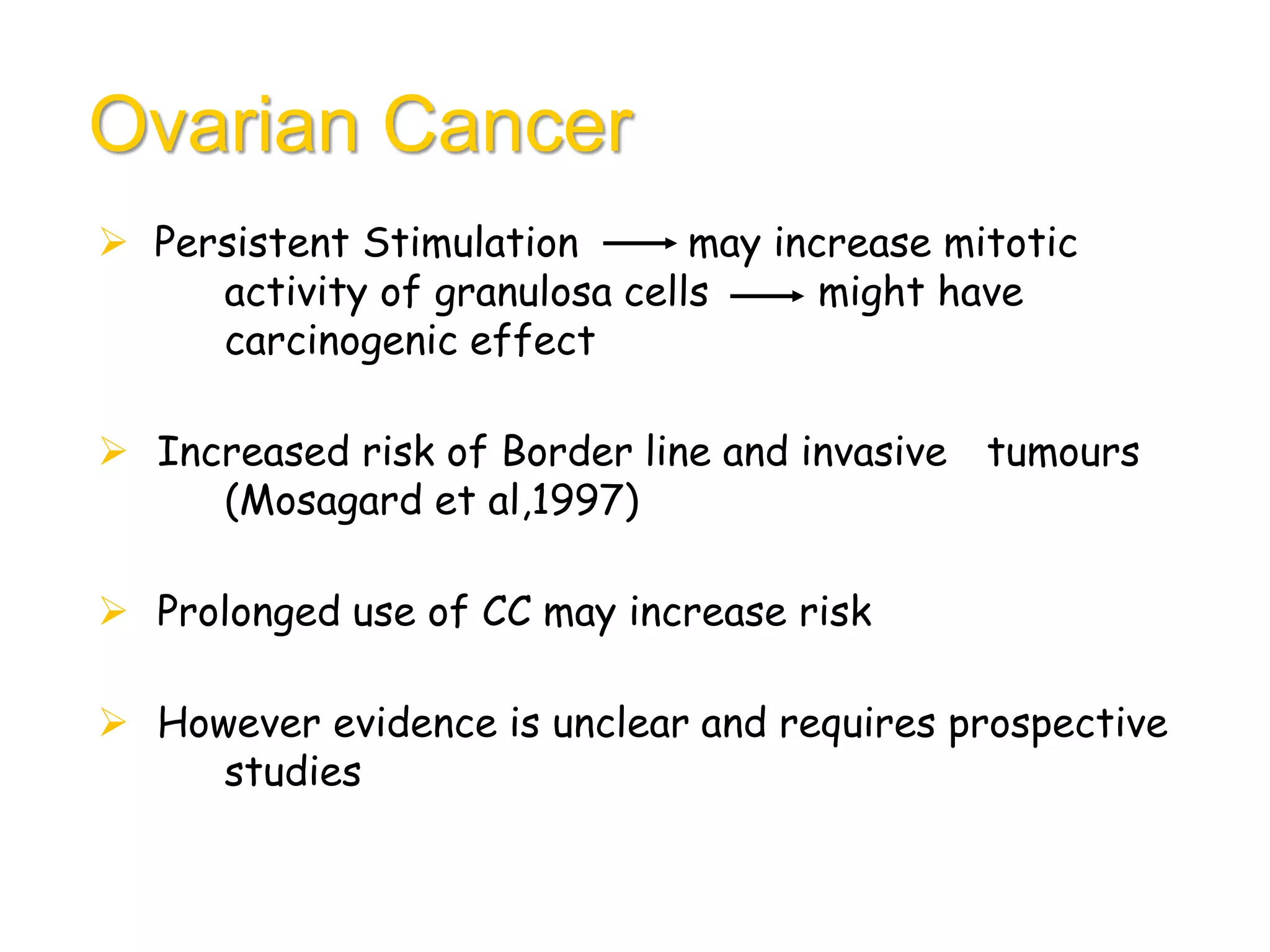
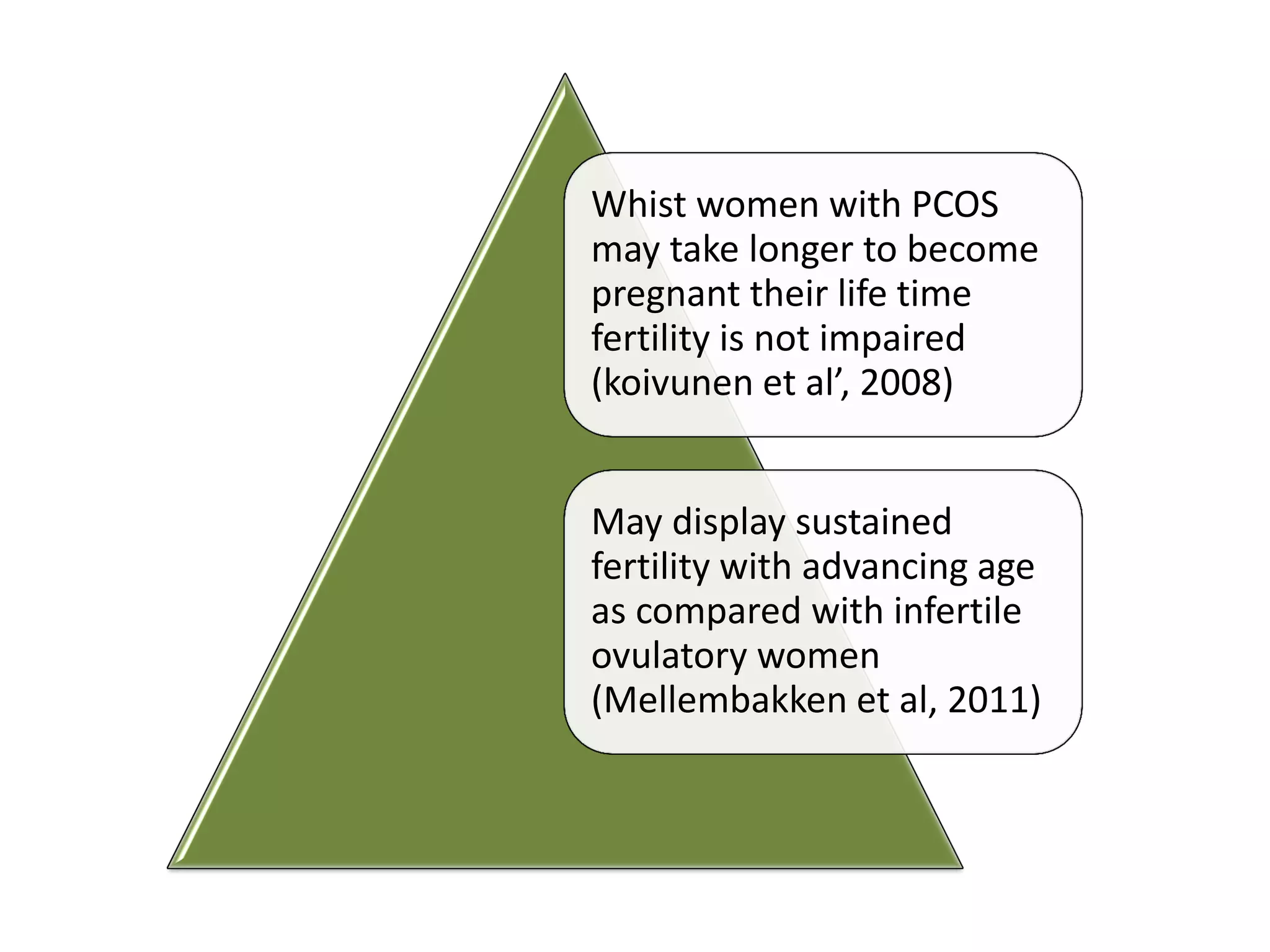
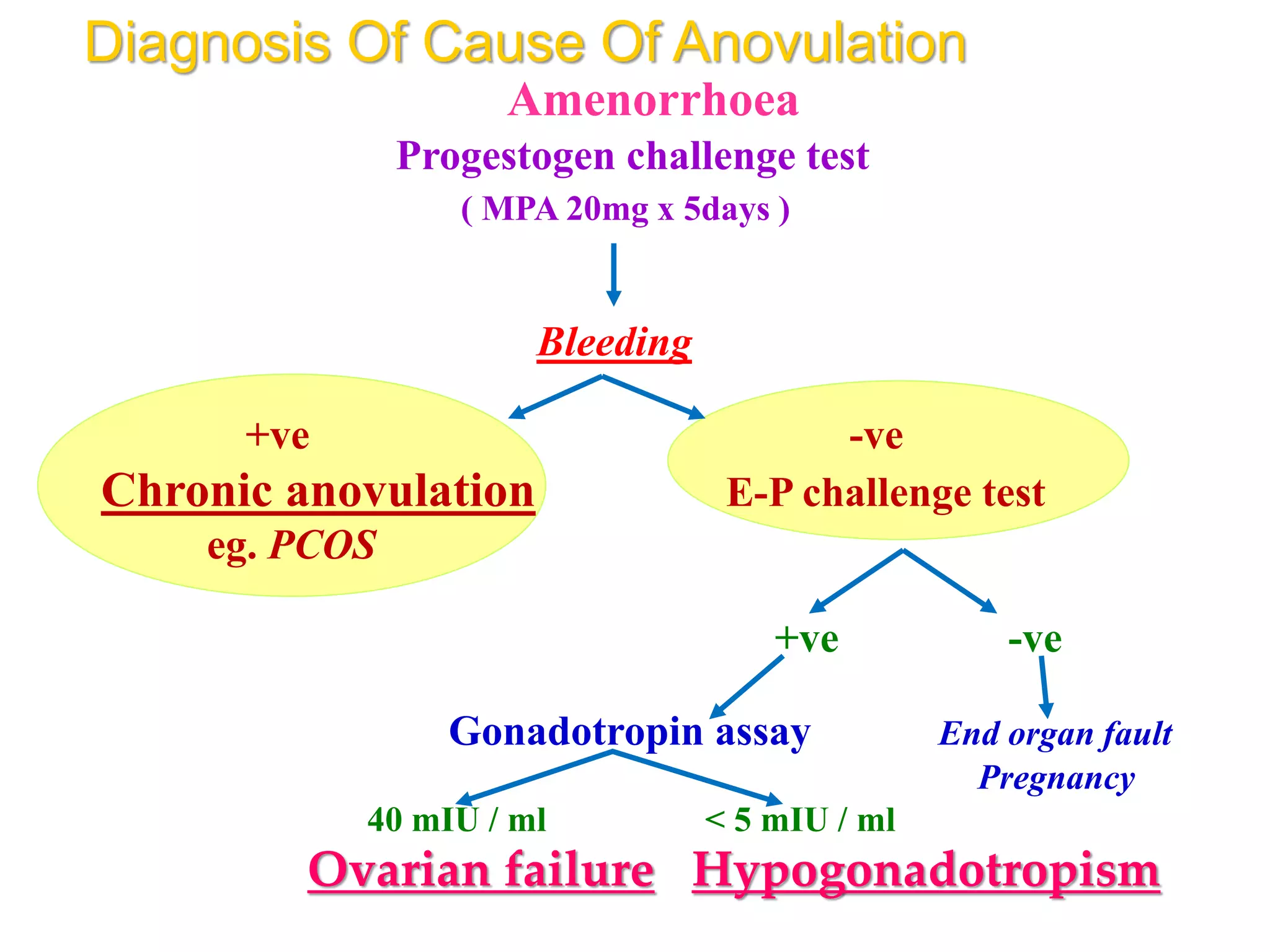
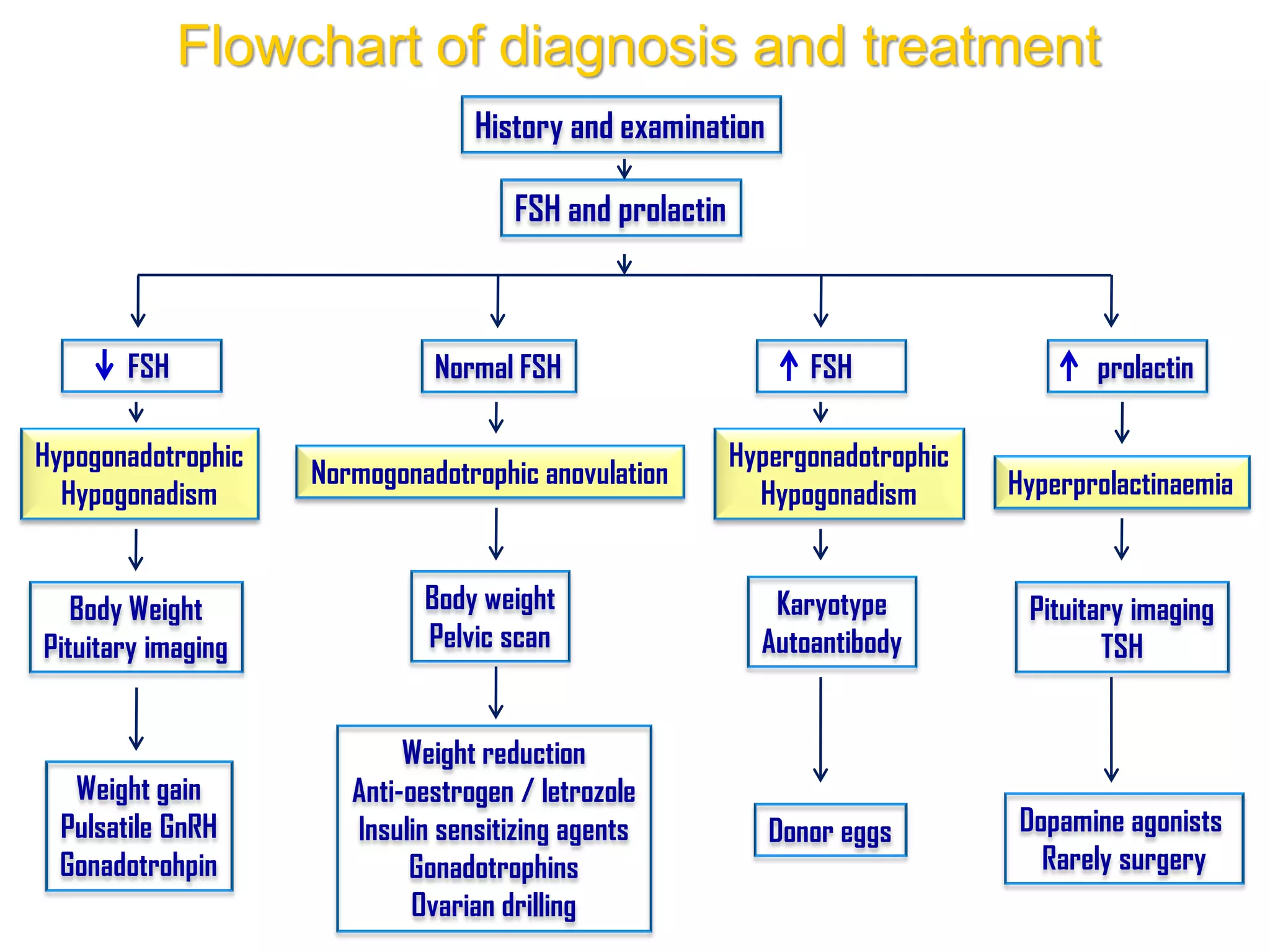
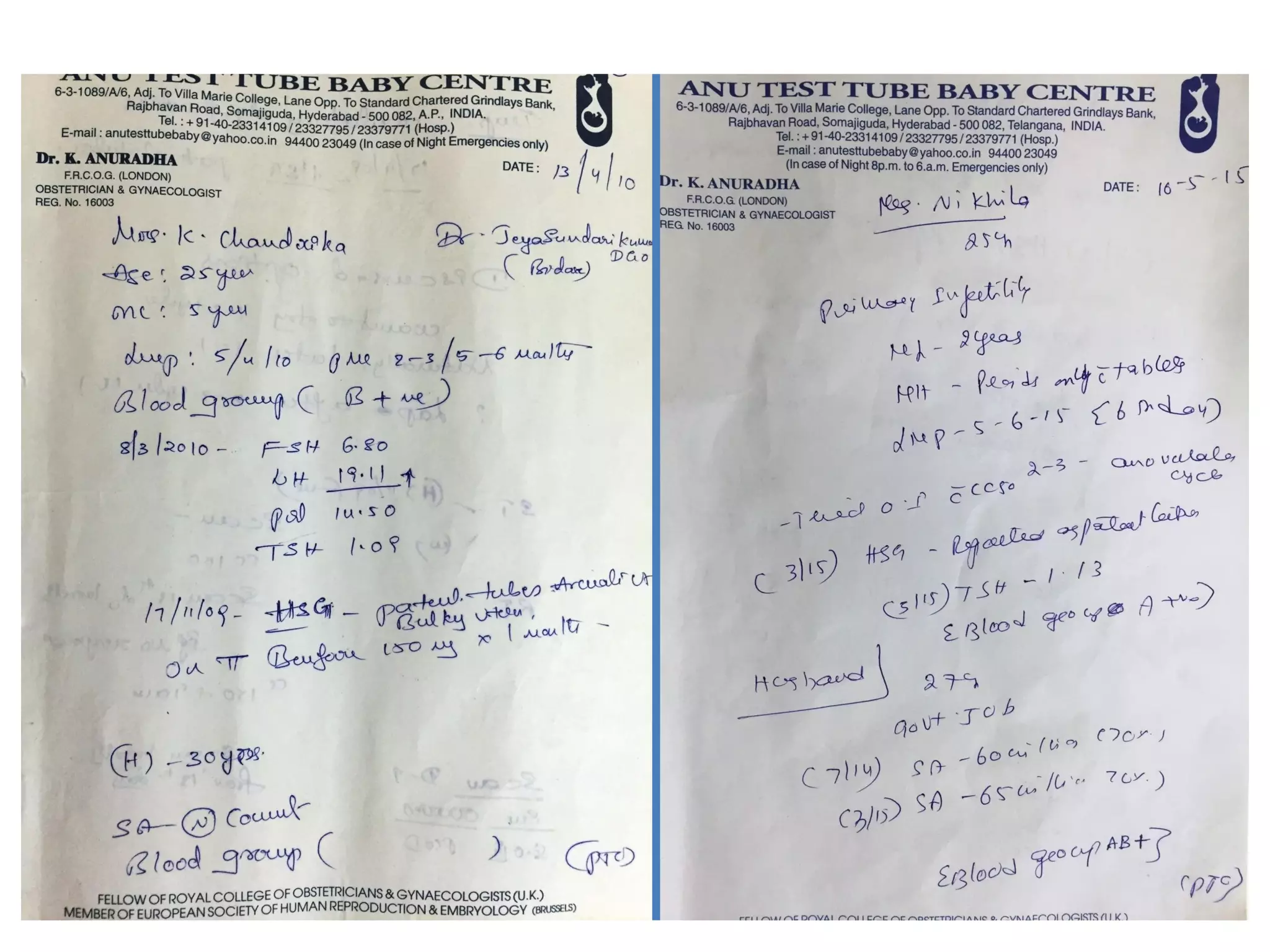
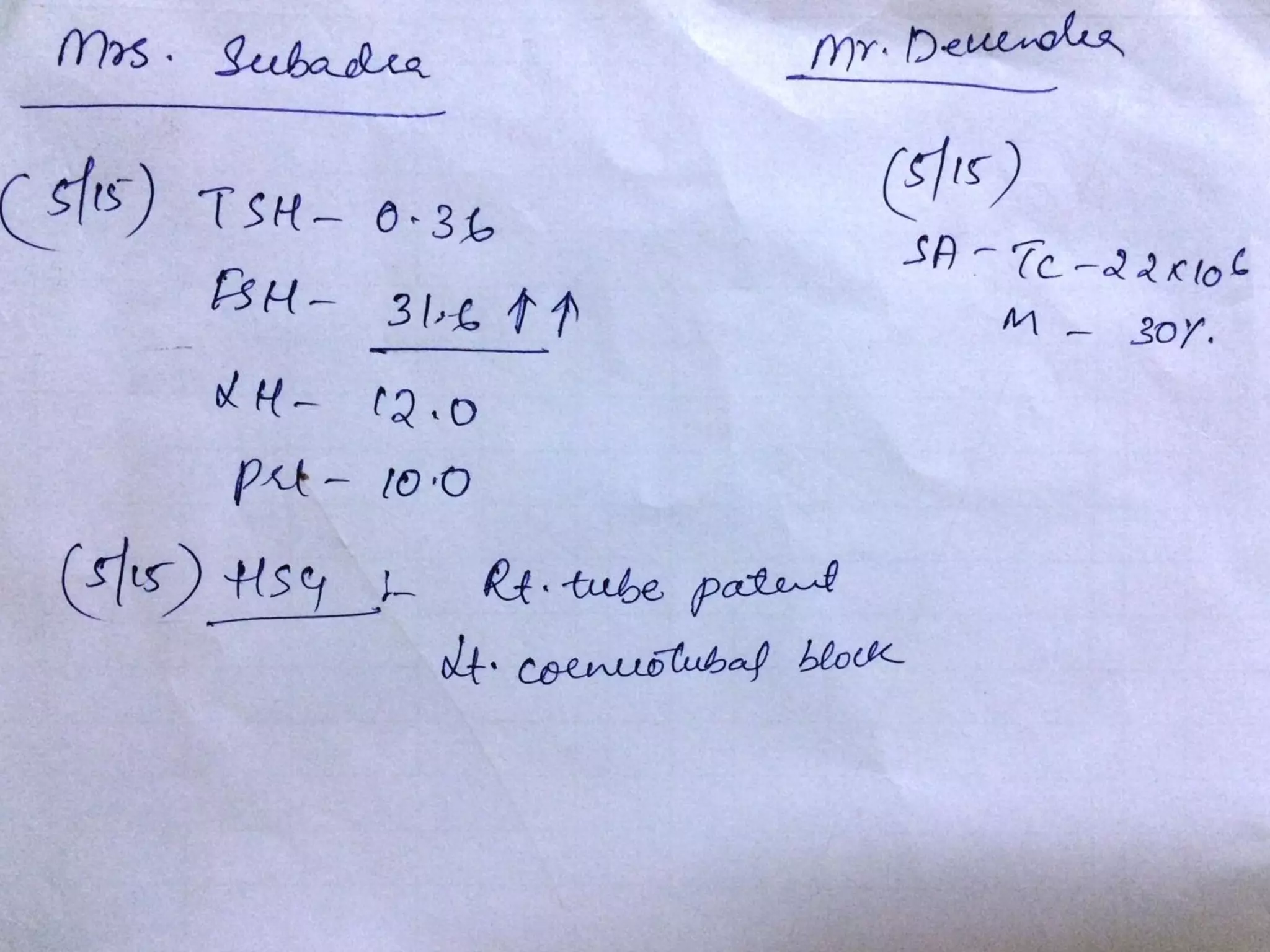
The document discusses ovulation induction (OI) protocols, highlighting the prevalence of anovulation as a significant cause of infertility among reproductive age couples, and outlines various treatment options tailored to specific etiologies of infertility. It emphasizes the necessity of precise diagnostic evaluations and the role of hormonal treatments, including the use of exogenous FSH and aromatase inhibitors like letrozole, in improving ovulation rates and overall fertility outcomes. The guidelines also recommend lifestyle modifications as a first-line approach and suggest that letrozole should be considered as a preferred treatment for patients with PCOS and obesity.