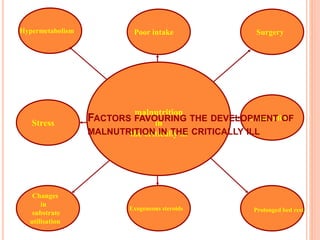
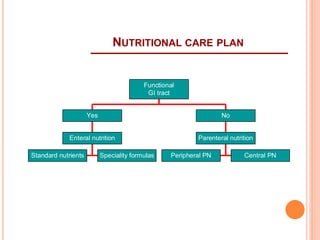
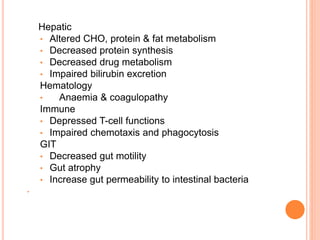
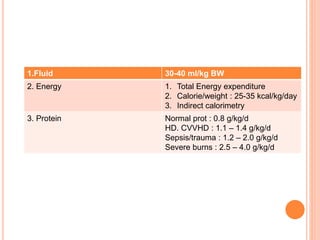
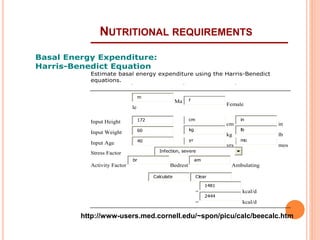
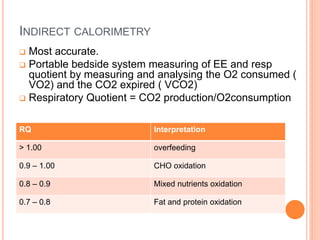
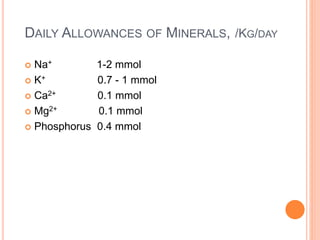
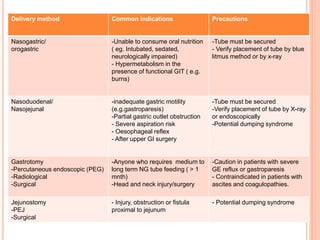
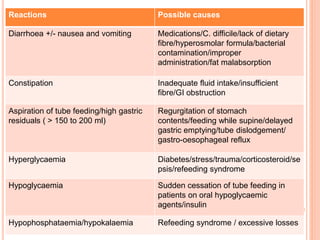
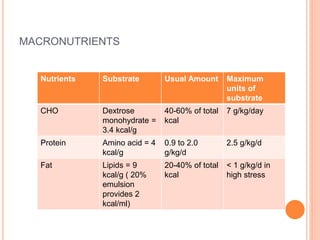
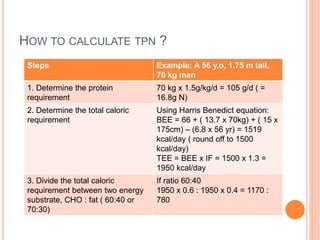
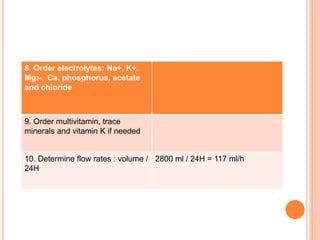
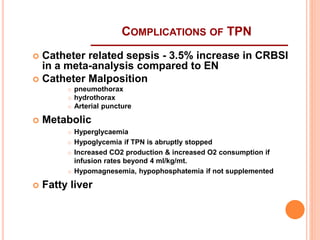
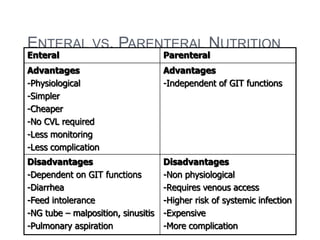
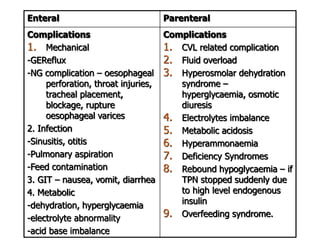
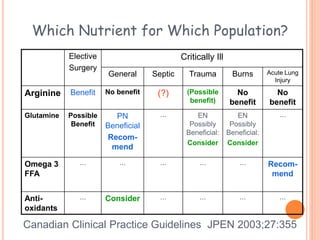
This document discusses nutrition support for critically ill patients in the intensive care unit (ICU). It provides a brief history of ICU nutrition and outlines the basis for nutritional support. Nutritional support is important to address the catabolism and malnutrition that often develops in critically ill patients. Enteral nutrition is preferred over parenteral nutrition when possible due to lower risks of infection and preservation of gut function. The document reviews nutritional requirements, supplementation, routes of administration including enteral and parenteral options, and potential complications of nutrition support.