1. The document discusses various medications used to treat central vertigo such as antihistamines, benzodiazepines, corticosteroids, and diuretics.
2. It provides dosing guidelines for adults and pediatrics for many of these medications.
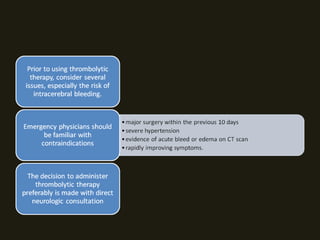
3. Most patients with central vertigo should be admitted to the hospital for further evaluation and treatment under a neurologist or neurosurgeon due to the serious nature of the underlying causes.