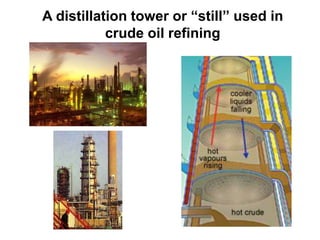
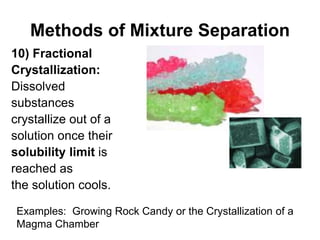
The document describes 10 methods for separating mixtures: 1) Mechanical separation uses physical properties like shape and color. 2) Magnetic separation uses magnetism. 3) Filtration uses differences in state of matter to separate solids from liquids. 4) Decanting separates liquids of different densities by pouring. 5) Distillation separates liquids based on differences in boiling point. 6-8) Other methods include evaporation, density separation, and using a centrifuge. 9) Paper chromatography separates mixtures using molecular polarity differences. 10) Fractional crystallization separates dissolved substances as a solution cools based on solubility limits. Videos and animations provide examples of many of these separation techniques.