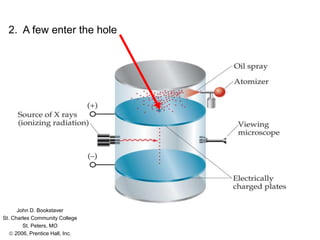
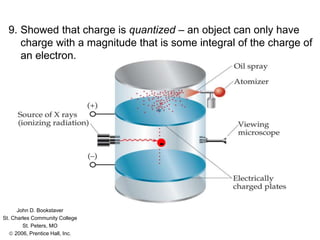
Millikan's oil drop experiment was conducted by Robert Millikan to determine the charge of an electron. Fine oil drops were sprayed between charged plates in an electric field. The field was adjusted to suspend individual drops, allowing their charge to be calculated based on balancing the electrical and gravitational forces. Millikan found that the drops' charges were always integer multiples of approximately 1.6×10−19 coulombs, demonstrating that electric charge is quantized in small whole-number values. This established the fundamental charge of an electron.



















![Storing Electric Energy
IV. Storing Electric Energy – The Capacitor
B. Capacitance (C) – ratio of charge to potential difference
http://www.jianghai.com/image/da2.jpg
C. Capacitor – device used to have a
specific capacitance (large capacitance
in small device)
V Voltage
Farad (F)C = q [=] Coulomb (C) =](https://image.slidesharecdn.com/millikan-200807163931/85/Millikan-23-320.jpg)
