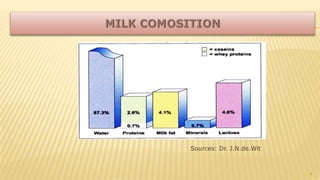
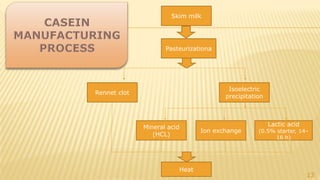
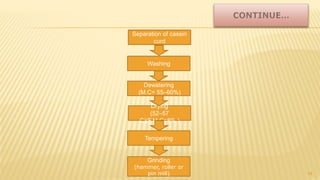
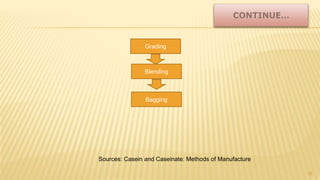
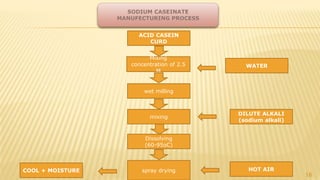
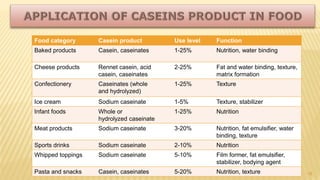
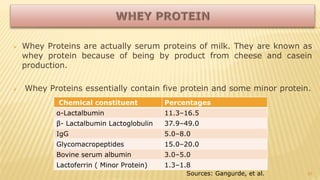
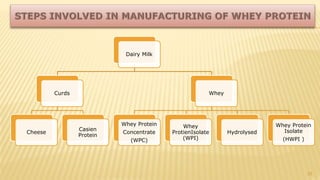
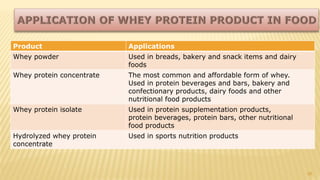
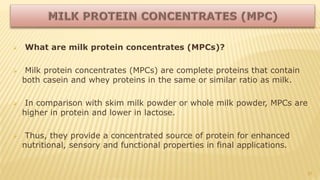
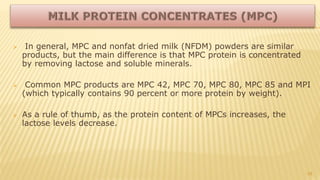
Milk proteins like casein and whey proteins are the primary proteins found in milk. Casein makes up around 80% of milk proteins and is further comprised of alpha, beta, and kappa casein. Whey protein makes up the remaining 20% and contains beta-lactoglobulin and alpha-lactalbumin. These milk proteins are separated through processes like cheese or casein production. They find various applications in food due to their functional properties such as water binding, emulsification, and foam formation. Common milk protein products include caseinates, whey protein concentrates, isolates, and hydrolysates which are used in foods like baked goods, dairy, meat products, and nutrition supplements.