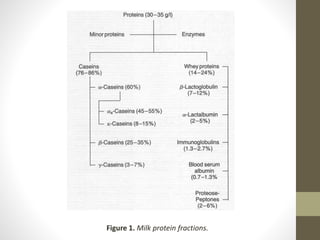
This lecture covers the chemistry of milk proteins, highlighting their importance in nutrition and life processes. It details the different types of milk proteins, including casein and whey proteins, their structures, functions, and how they can be separated. Additionally, it discusses the presence of enzymes in milk and their roles, including their significance in pasteurization and preservation.