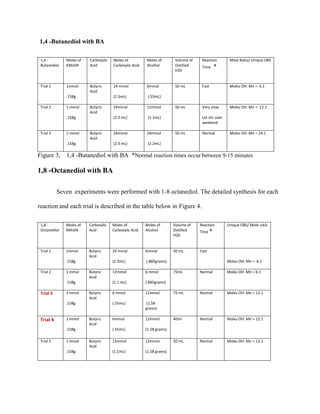
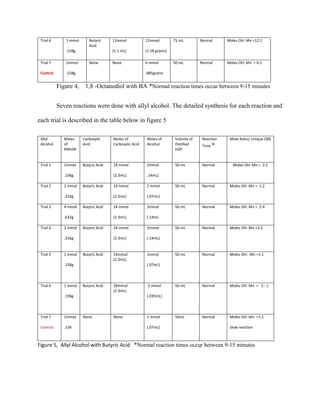
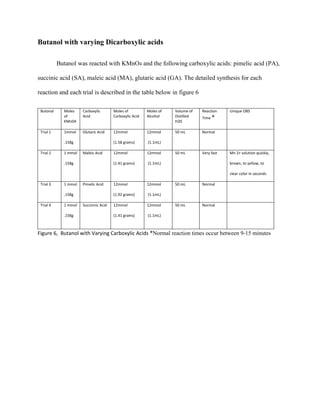
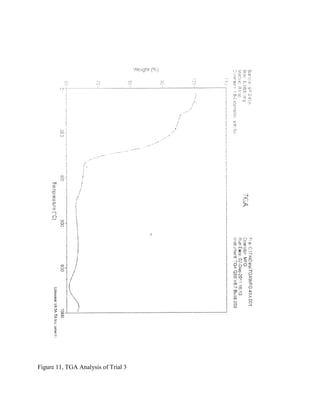
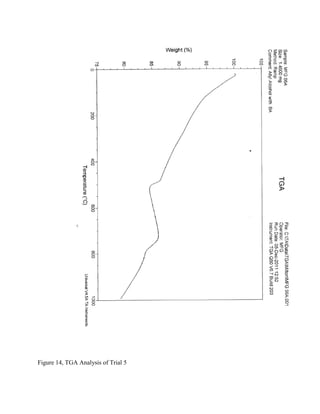
The document summarizes research into synthesizing manganese oxide nanoparticles through various chemical reactions. It describes reacting potassium permanganate with alcohols and di-alcohols in the presence of carboxylic acids. A series of experiments were conducted using different alcohols, di-alcohols and carboxylic acids. The experiments aimed to produce nanostructured materials with interesting morphology, small particle size, and high surface area. Scanning electron microscopy images of some products showed spheres and nanostructures, though not all reactions yielded useful materials. The research seeks to develop manganese oxide materials for applications in batteries, catalysis and toxic waste removal.