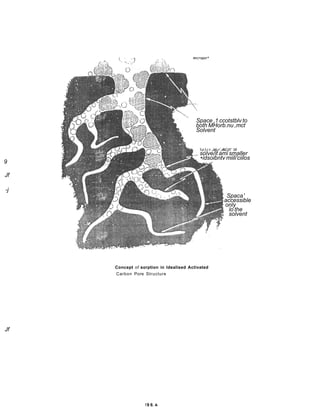
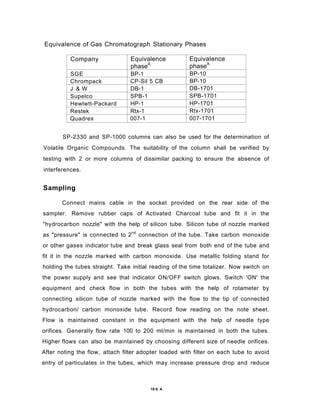
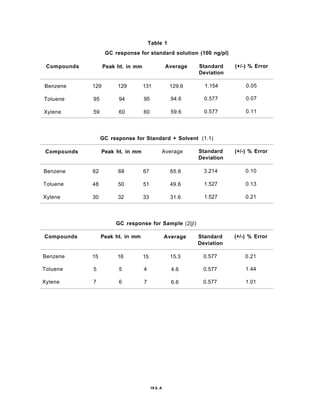
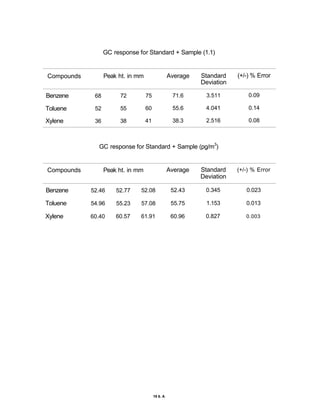
The document discusses activated carbon and its application for monitoring volatile organic compounds. It provides details on:
1) The preparation of activated carbon involves carbonizing raw materials like wood, coal and shells, followed by physical or chemical activation to increase surface area and porosity.
2) Granular activated carbon is characterized by its porous structure and surface chemistry, which contains various functional groups that influence adsorption properties.
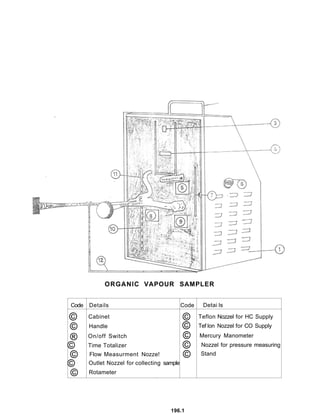
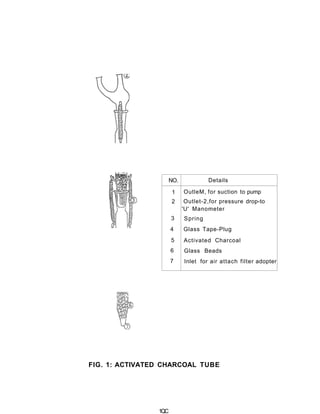
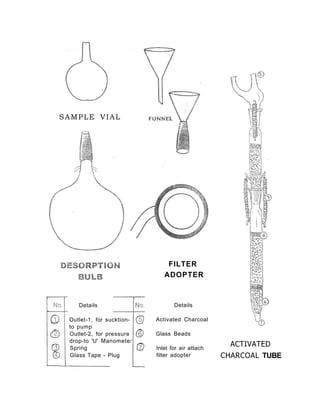
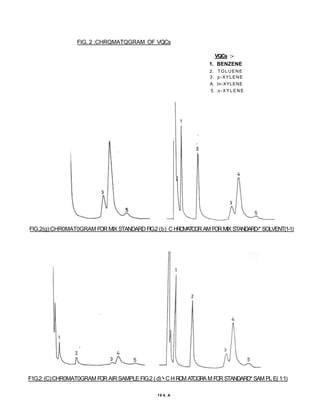
3) Volatile organic compounds can be monitored by passing an air sample through an activated carbon tube. Adsorbed compounds are then desorbed and analyzed using gas chromatography.