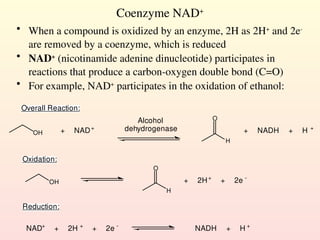
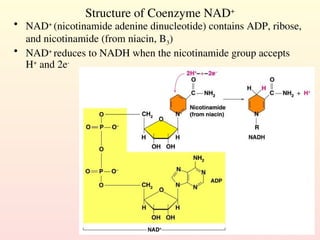
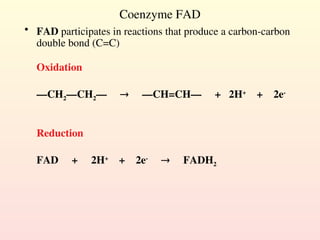
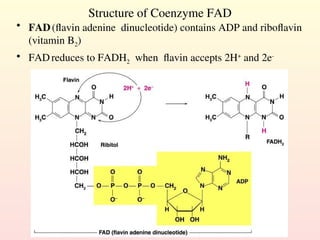
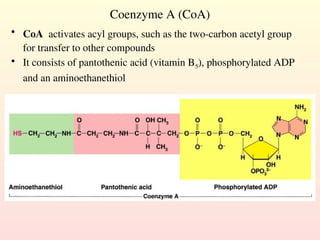
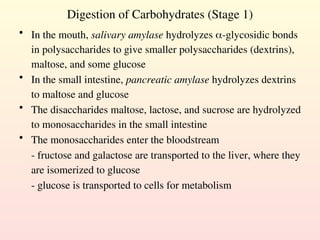
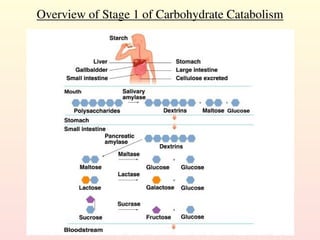
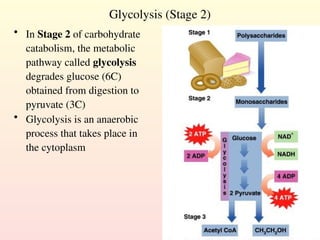
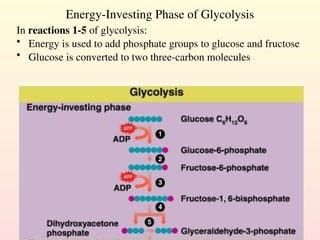
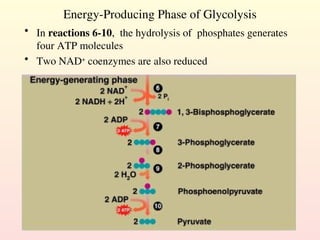
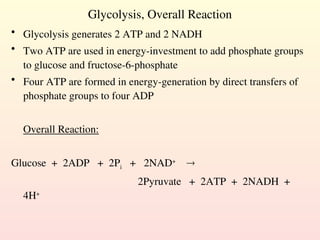
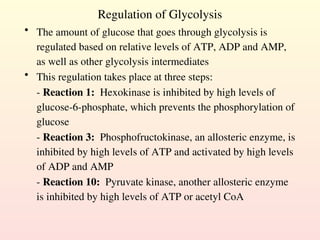
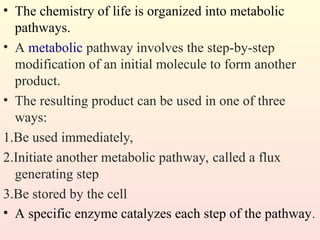
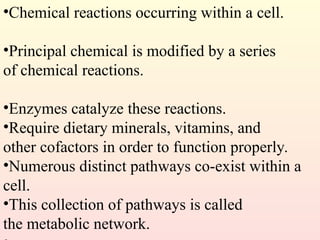
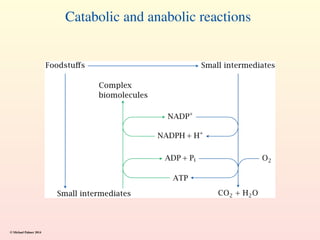
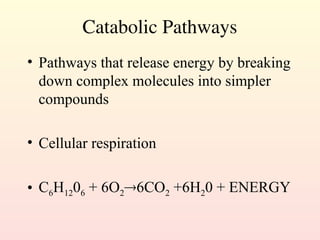
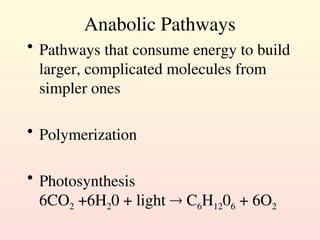
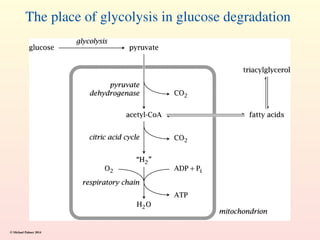
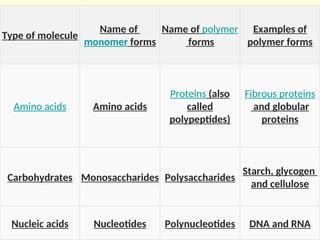
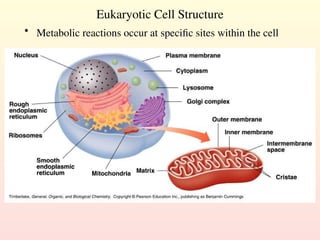
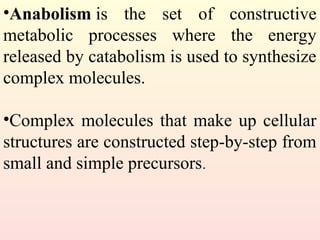
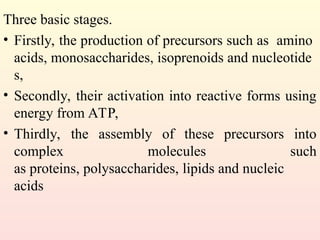
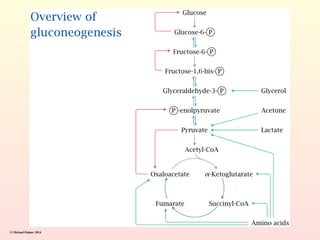
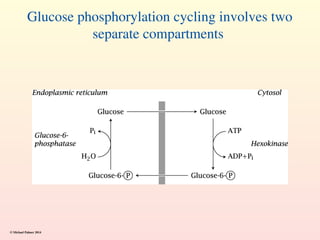
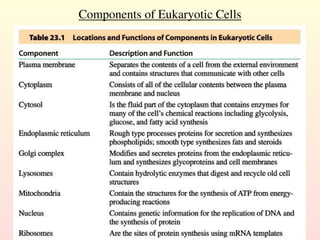
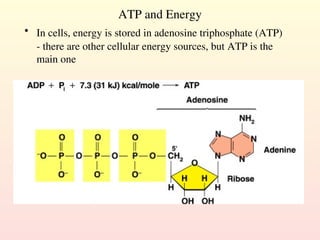
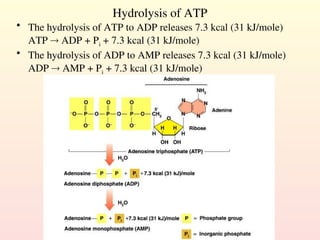
Metabolism encompasses all chemical processes in an organism and is organized into metabolic pathways, which involve converting molecules to produce various substances. These pathways include catabolic reactions that break down molecules for energy and anabolic reactions that synthesize complex molecules from simpler ones, which are crucial for maintaining homeostasis. Metabolic rate, influenced by factors such as age, sex, and physical activity, measures the energy released from food catabolism and is essential for bodily functions.











![ATP and Muscle Contraction
• Muscle fibers contains
filaments of actin and
myosin
• When a nerve impulse
increases [Ca2
+], the
filaments slide closer
together to contract the
muscle
• The hydrolysis of ATP in
muscle provides the
energy for contraction
• As Ca2+
and ATP decrease,
the filaments return to the
relaxed position](https://image.slidesharecdn.com/metabolism3-250123051031-e77eec19/85/metabolism-3-ppt-powerpoint-presentation-33-320.jpg)