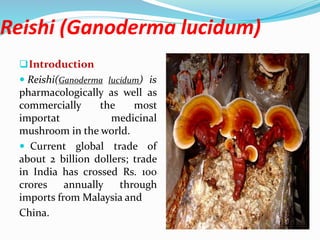
The document discusses the cultivation of four medicinally important mushrooms - Reishi, Shiitake, Oyster and Maitake mushrooms. It describes the substrates, spawning and fruiting conditions required for successful cultivation of each mushroom. Precautions during cultivation like maintaining proper temperature, humidity and avoiding contamination are also summarized.