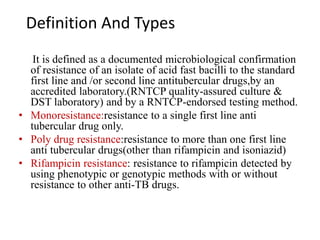
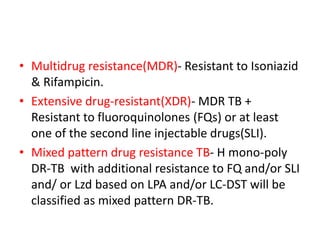
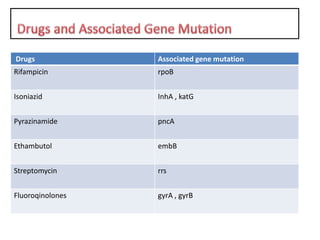
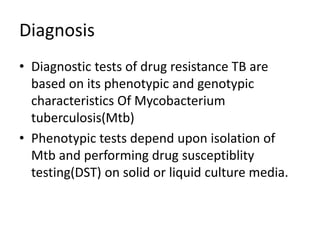
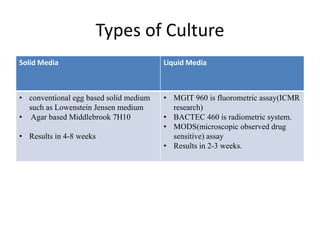
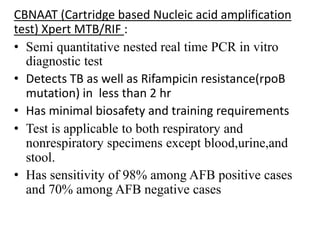
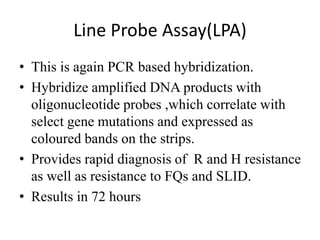
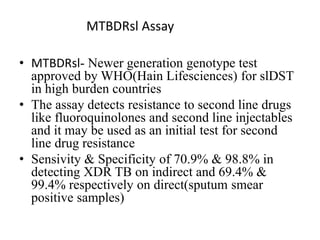
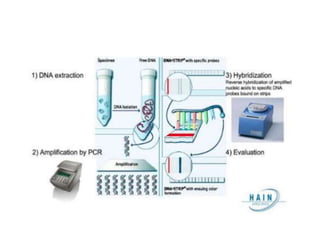
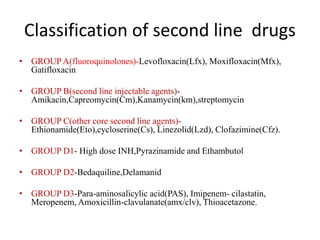
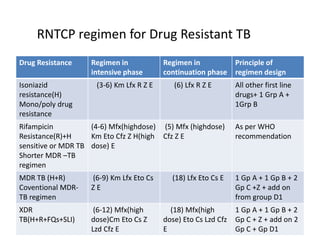
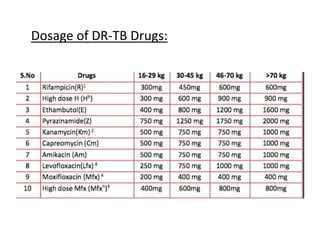
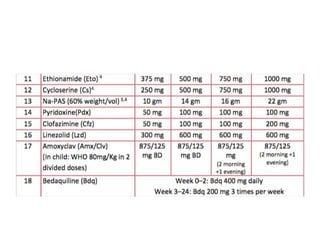
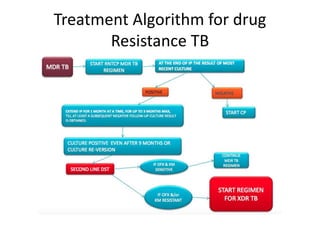
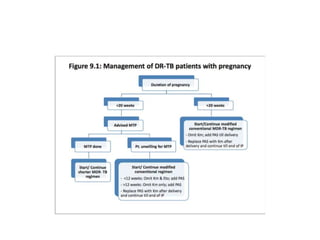
Drug resistant tuberculosis is defined as resistance of Mycobacterium tuberculosis to antitubercular drugs. It can be multidrug resistant (MDR), extensively drug resistant (XDR), or have other resistance patterns. Diagnosis involves culture and drug susceptibility testing using solid or liquid media, as well as molecular tests like CBNAAT and line probe assays. Treatment requires specialized regimens using second line drugs for longer periods. Patient follow up assesses treatment response through repeated sputum cultures. Newer drugs like bedaquiline and delamanid are being added to treatment regimens to improve outcomes for drug resistant tuberculosis.