Multi Drug Resistant - Tuberculosis
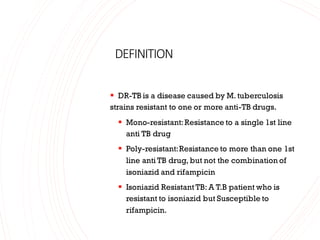
- 1. DEFINITION ▪ DR-TB is a disease caused by M. tuberculosis strains resistant to one or more anti-TB drugs. ▪ Mono-resistant:Resistance to a single 1st line anti TB drug ▪ Poly-resistant:Resistance to more than one 1st line anti TB drug, but not the combination of isoniazid and rifampicin ▪ Isoniazid Resistant TB: A T.B patient who is resistant to isoniazid but Susceptible to rifampicin.
- 2. ▪ Multi-drug-resistant tuberculosis (MDR-TB) is defined as resistance to rifampicin and isoniazid,with or without resistance to other first-line anti-TB drugs. ▪ Rifampicin Resistant T.B (RR-TB):ATB patient whose biological specimen is resistant to Rifampicin,detected by using phenotypic & genotypic methods with or without resistance to other anti TB drugs. It include any resistance to R in the form of mono resistance, poly resistance,MDR TB & XDR TB.
- 3. ▪ Extensively drug-resistant tuberculosis (XDR-TB) is defined as esistance to rifampicin,isoniazid,any fluoroquinolone and resistance to one or more of the following injectable anti-TB drugs: kanamycin,amikacin and capreomycin. ▪ Latent Tuberculosis : A state of persistent immane response to stimulation by Mycobacteria tuberculosis antigens with no evidence of clinically manifest active T.B.
- 4. ▪ Presumptive DR-TB:It refers to patient who is eligible for rifampicin resistance screening at the time of resistance or during the course of treatment for DS-TB or H mono/poly DR-TB. ▪ Drug susceptibility testing (DST):It refers to in vitro testing using either of the phenotypic methods to determine susceptibility. ▪ Drug resistance testing (DRT):It refers to in- vitro testing using genotypic methods (molecular techniques) to determine resistance.
- 5. ▪ WHO estimates that there were about 4,50,000 new(incident) mdr-tb cases in the world in 2012. ▪ Morethan onehalfofthese casesoccurred inCHINA,INDIA &the RUSSIAN FEDERATION. ▪ Enrolments on MDR-TB treatment in2012: were equivalent toone in four of themdr-tb casesestimated tooccuramongpulmonaryTBpatientsnotifiedin the world. ▪ Treatment success:48%ofpatientswithMDR-TBenrolled ontreatment in 2010 were reported tohavebeensuccessfullytreated ▪ WHO estimates that thereareabout650,000MDR-TBcasesintheworldatany ▪ onetime. ▪ Onlyasmallproportion ofthese casesaredetected and treated appropriately giventhatmany lowandlowermiddle-incomecountries stilllacksufficient diagnostic capacity todetect MDR/XDR-TB. Epidemiology
- 6. MDR-TB • Globally Prevalence of MDR- TB3.4% in new TB patients20% in those previously treated • By October 2011, 77 countries had reported at least one case of XDR. • Coverage is low particularly in the African continent as a result of low capacity for testing for second-line medicines. • WHO estimate that some 5% of people with multidrug resistant TB may actually have extensively drug resistant Problem Statement
- 10. Durg resistancein Tb ▪ Drug-resistant TB is caused by M. tuberculosis organisms t ha t are resistant t o the drugs normally used t o treat the disease. Drug- resistant TB is transmitted in the same way as drug-susceptible TB, and is no more infectious than drug-susceptible TB. However, delay in the recognition of drug resistance or prolonged periods of infectiousness may facilitate increased transmission and further development of drug resistance.
- 11. ❑Drug resistant TB ❑ Mono resistance ❑ Poly resistance ❑ Multi Drug Resistant TB(MDR-TB) ❑ Extensive Drug Resistant TB (XDR-TB) ❑ Total Drug Resistance (TDR-TB)
- 12. •Mono Drug Resistance (Resistance to single first line ATT) • Poly Drug Resistance (Resistance to two or more first line ATT except MDR-TB) • Multi-drug resistant tuberculosis (MDR TB) is defined as resistance to isoniazid and Rifampicin (a laboratory diagnosis). •Extensively drug resistant TB (XDR-TB) is MDR +resistance to any fluoroquinolone + resistance to at least one 2nd-line injectable drug (amikacin, kanamycin, or capreomycin •Resistance to all first-line anti-TB drugs (FLD) and second-line anti-TB drugs (SLD) that were tested.
- 14. Mechanism of drug resistance 1)Cell wall: The cell wall of M. tuberculosis consists of complex lipids, an d it acts as a permeability barrier from drugs. 2)Drug modifying & inactivating enzymes: The enzymes usually phospho rylate, acetylate, or adenylate the drug compounds. 3)Drug efflux systems 4)Mutations: Spontaneous mutations in the M. tuberculosis genome can give rise to proteins that make the bacterium drug resistant, depending on the dru g action.
- 16. FACTORSRESPONSIBLEFOR DEVELOPMENTOFDRUGRESISTANCE ▪ Clinical / Operational factors ▪ Unreliable treatment regimen by doctors ▪ Lesser number of drugs ▪ Inadequate dosage / duration ▪ Addition of a single drug in failing regimen ▪ Easy availability of drugs in private sector ▪ Poor drug supply ▪ Poor quality of drugs:poor bioavailability
- 17. Biological factors ▪ Initial bacillary population ▪ •Local factors in host favourable for multiplication of bacilli ▪ Presence of drug in insufficient concentration ▪ Irregular intake ▪ inadequate duration ▪ Neglect of disease ▪ Ignorance
- 18. ▪ Most cases of acquired MDRTB are due to inappropriate treatmentwith a single antiTB drug, usuallyINH.This can occur due to a medical provider, such as a doctor or nurse,improperlyprescribing, ineffective treatment,but may also be due to the patient not taking the medication correctly,whichcan be due to a variety of reasons,including expenseor scarcity of medicines,patientforgetfulness,or patient stopping treatment early becausethey feel better.
- 19. WhoisatriskforgettingMDRTB? ▪ Drug resistance ismore common in people who: ▪ Do not take their TBmedicine regularly ▪ Do not take all of their TB medicinesas told by their doctor or nurse Develop active TB disease again, after having taken TB medicine in the past ▪ Come from areas of the world where drug-resistant TB is common ▪ Have spent time with someone known to have drug- resistant TB disease
- 20. Complications ofPulmonary Tuberculosis 1. Hemoptysis 2. Pleurisy 3. Pleural effusion 4. Empyema 5. Pneumothorax 6. Aspergilloma 7. Endobronchitis 8. Brochiectasis 9. Laryngitis 10. Cor pulmonale 11. Ca bronchus 12. Enteritis 13. Miliary Tuberculosis
- 21. ▪ A variety of complication occur in pulmonary tuberculosis.They can be categorised as follows :- ▪ ▪ (1) Parenchymal lesions ▪ •Thin wall cavity ▪ •Aspergilloma ▪ •End stage lung destruction ▪ •Scar carcinoma ▪ ▪ (2) Airway Lesions ▪ • Tuberculous laryngitis ▪ • Bronchiectasis ▪ •Tracheobronchial stenosis ▪ •Broncholithiasis ▪ • Anthracofibrosis ▪ ▪
- 22. ▪ (3) Vascular lesions ▪ •Rasmussen aneuryum ▪ ▪ (4) Pleural lesions ▪ • Dry pleurisy ▪ •Pleural effusion ▪ •Empyema & bronchopleural fistula ▪ • Pneumothorax ▪ ▪ (5) General Complications. ▪ •Cor- pulmonale ▪ •Secondory amyloidosis ▪ •Chronic respiratory failure
- 23. MDR TB
- 24. The following diagnostic technologies are currently available under RNTCP and recommended for diagnosis of MDR-TB . MDR-TB diagnostic technology Choice. First-Molecular DST [e.g. cartridge-based automated nucleic acid amplification test (CBNAAT) or line probe assay (LPA)] Second-Liquid culture isolation and LPA DST Third-Solid culture isolation and LPA DST Fourth-Liquid culture isolation and Liquid DST Fifth-Solid culture isolation and DST
- 25. LineProbeAssays: A DNA strip test that allows simultaneous molecular identification of tuberculosis and the most common genetic mutations causing resistance to rifampicin and isoniazid that is rpoB gene conferring rifampicin resistance and mutations on the katG gene which is associated with higher levels of isoniazid resistance and inhA gene mutations which is associated with lower levels of isoniazid resistance. These tests have been approved for direct testing on smear positive specimens and on isolates from solid and liquid culture. In 2008,theWHO issued a recommendation for the use of molecular LPA for the rapid diagnosis of MDR-TB in high TB-burden,low-income settings. The test that is available in the country is the Genotype MTBDRplus assay which is a PCR based hybridisation assay.
- 26. Advantages of the test are that: It detects MTB and resistance to RIF & INH at the same time from one specimen. It reduces time to diagnosis of MDR-TB to 7 days. Cost-effective when compared with TB culture and DST. They also demonstrated significant patient benefits, including early targeted treatment of MDR-TB and the potential interruption of transmission.
- 27. Thelimitations ofthe test are: It cannot be used for monitoring patientson treatment because it does not distinguish between live and dead bacilli, therefore its use is limited to diagnosis It is dependent on smear results, can only be performed on smear positive or culture-positive sputum specimen Labour intensive Prone to contamination and human error Requires a lot of space - at least 3 separate rooms for the different steps. A small proportion of resistance detected may not correlate with physiological resistance. NEW Version 2 of the MTBDRplus which can be used on smear positive and negative sputumspecimensis available and currently being validated in the country. MTBDRsl isavailable for second line testing. This test may be used as a rule in test for XDR-TB in high risk groups.
- 28. This is a heterogeneousgroupof tests thatuse either the polymerasechainreaction (PCR) techniqueor Transcription mediatedamplification (TMA)or other forms of nucleicacid amplificationmethodsto detect mycobacterial nucleicacid. These test vary in which nucleicacidsequencethey detect and vary in their accuracy. sensitivity92% specificity99%
- 29. Xpert® MTB/RIF (CBNAAT) The test is called Xpert MTB/RIF also called as cartridge based nucleic acid amplification test . The instrument is a GeneXpert (GXP). GeneXpert is an automatedmolecular platform to detect M. tuberculosis and rifampicin resistance testing by targeting specific mutationsin the rpoB gene. It is approved for use directlyon raw sputum and resultsshould be available within 2 hours in the laboratorybut available in healthfacilities within 48 hours. The test involves only three manual steps: The addition of sample treatment reagent to liquefy and inactivate the sputum Transfer of 2ml of liquefied sputum to the cartridge Loading the cartridge into the device for the assay
- 30. Advantages of the test are : It detects MTBand Rifampicin resistance from one specimen at the same time. Resultsare available in approx. 2 hours. It can also be used on the following processed samples- CSF,aspirates (gastric, lymph node) and tissue (i.e. pleural biospy). It is specificfor MTB complex The limitations of this test are : It cannot be used for monitoring treatment because it does not distinguish between live and dead bacilli, its use is therefore limited to diagnosis A small proportion of Rifampicin resistance detected may not correlate with physiological resistance (leading to discordancebetween Xpert and DST results or clinical outcome) The assay is semi-quantitative and definesa positive test as “very low”, “low”, “medium”, and “high”. Thisgrading is not reported on the laboratory result. There is no direct correlation between the Xpert semi-quantitative result and the smear grading of scanty, +, ++ and +++. The rifampicin resultscan only be reported if MTB complex is detected.
- 31. In 1969, Deland and Wagner developed a techniquefor semi-automateddetection of the metabolism of bacteria by measuringthe 14CO2 liberated duringthe growth and decarboxylationof 14C-labeled substrateincorporated in the growth medium. This radiometric techniquewas widelyused for blood culture using the BACTEC 460 instrument. In 1980, this technique was introduced commercially for mycobacterialrecoveryfrom clinical specimens and drug susceptibility testing. One of the disadvantages of the BACTEC 460 TB System is the use of 14C-Labeled radioactive substrate.Because of the strict regulations of handlingand waste disposal ofradioactive material, it became necessary to develop a non-radiometric technique for mycobacterialculture and susceptibility testing. Becton, Dickinson and Company (BD) developed a new system called Mycobacteria Growth Indicator Tube (MGIT™), which is non-radiometric and offers the same rapid, sensitive and reliable methods of testing as the BACTEC 460 TB System. BBL MGIT™ System is the manual systemwhileBACTEC MGIT 960 (MGIT 960) is the fully automatic systemfor detection of mycobacterial growth and drug susceptibilitytesting of M. tuberculosis
- 32. Principle of the BACTEC MGIT System Manual MGIT medium. The MGIT contains 7.0ml of modified Middlebrook 7H9 broth base. In addition to Middlebrook 7H9 liquid media, the MGIT tube contains an oxygen-quenched fluorochrome, tris 4, 7- diphenyl- 1, 10 - phenonthroline ruthenium chloride pentahydrate, embedded in silicone at the bottom of the tube. During bacterial growth within the tube, the free oxygen is utilized and is replaced with carbon dioxide. With depletion of free oxygen, the fluorochrome is no longer inhibited, resulting in fluorescence within the MGIT tube when visualized under UV light. The intensity of fluorescence is directly proportional to the extent of oxygen depletion and is indicative of the number of bacilli present. MGIT tubes may be incubated at 37ºC and read using the manual transillumination with a 365nm UV light.
- 33. MDR-TBinspecial situations Pregnancy and lactation HIV-TB co-infection renal impairment Pre-existing liver diseasE Seizure disorder Psychiatric illness
- 34. MDR-TB During pregnancy and lactation
- 35. MDR-TBIN PLHIV The shorter or longer oral MDR-TB regimen can be used in PLHIV, including those who are receiving ART. In PLHIV with pulmonary MDR/RR-TB, additive toxicities or drug-drug interactions between anti-TB and ART medicines potentially overlap e.g. Mfx and Cfz or Efavirenz and Bdq, ritonavir may potentially increase the risk of Bdq related adverse events and hence combined use should be avoided or used with caution
- 37. MDR -TB in pre- existing liver disease In general,most second-line drugs can be safely used in the presence of mild hepatic impairment, as They are relatively less hepatotoxic than first- line drugs. patients with pre-existing liver disease with persistently abnormal liver function test, a shorter oral MDR/RR-TB regimen will be avoided due to presence of H(h), Eto and Z.
- 38. Seizuredisorders
- 39. PREVENTIVETREATMENT OFCONTACTSOF DR-TB MDR-TBisa seriousformof TB and islesseasyto treat than other typesof TB disease. HHC of patients with MDR/RR-TBor H mono/poly DR-TBare at higher risk of TBI than contacts exposed to drug-sensitive TB, however the risk of progression to TB disease does not differ among contacts in both groups. Recent evidence fromsystematic review & meta-analysis, andcost-effectiveness of treatment of latent tuberculosis to reduce progressionto multidrug-resistant tuberculosis revealed. A reduced risk of TBincidence with treatment for MDR-LTBI, suggesting effectiveness in prevention of progressionto MDR-TB, and confirmed cost- effectiveness; Estimated MDR-TBincidence reduction was 90% (9%-99%) using data from5 comparison studies; High treatment discontinuationrates due to adverse effects in persons taking pyrazinamide containing regimens; and Cost-effectiveness was greatest using a fluoroquinolone/ethambutol combination regimen.
- 40. WHOrecommendationsonTPTamongcontactsofDR-TB patients WHO recommends TPT among contacts exposed to MDR- TB with FQ sensitive or H resistant with R sensitive DR-TB patients following consideration of intensity of exposure; confirming the source patient and her/his drug resistance pattern confirmed bacteriologically and ascertaining TBI using IGRA or TST. Among contacts exposed to patients with known MDR-TB with FQ sensitive, WHO suggeststhe use of levofloxacin for six months (pediatric formulation for child contacts) if tolerated. If H susceptibility is confirmed in RR-TB index patients, contacts may be given 6H. Among contacts exposed to individuals with known H-resistant TB with R sensitive, the use of rifampicin for four months is proposed. Regardless of whether treatmentis given or not, clinical followup should be done for two years and any emergent signs and symptoms suggestive of TB should be actively investigated and curative regimens started as needed.
- 41. ONGOINGSTUDIES Randomizedcontrolledtrials onMDR-TB preventive treatment are urgently needed to improve the evidence base results from following three RCTs of TPT among HHC ofMDR-TB patients are expected to become available in the next few years: TB CHAMP.Testingsix months of levofloxacin (Lfx) vs placebo in infants and young children less than five years of age exposed to MDR-TB (South Africa; ongoing recruitment and intending to publish by end 2021). V-QUIN. Testing 24weeks of Lfx vs placebo in all ages with evidence of infection (Viet Nam; recruitment completed; date of ending data collection is March 2022). PHOENIx. Testing26 weeks of delamanid vs isoniazid in all ages (11 countries; estimated completion in mid-2025).
- 42. ALGORITHM FOR SCREENING AND RULING OUT ACTIVE TB AM ON G HHC OF DR-TB PATIENTS
- 43. SALIENT FEATURES OF THE INTEGRATED ALGORITHM ● Once a DR-TB patient is identi ed, all HHCs are counselled, screened and evaluated to rule out active TB. ● NAAT will be used upfront among contacts with symptoms or abnormal chest X-ray to diagnose TB. ● If the result is MTB detected with no resistance, the treatment for DS-TB is initiated; ● If the result is MTB detected with H and/or R resistance, manage as per DR-TB guidelines; ● If the result is MTB not detected, in HHC <5 years, assess for TPT and check for any contraindications. ● If the result is MTB not detected, in HHC >5 years of age with TBI test positive or unavailable and chest X-ray is normal or unavailable check for any contraindications; ● If contraindications to TPT drugs exists, defer TPT and if no contraindication exists, offer TPT regimen as appropriated based on DST pattern of the index patient; and ● Follow-up for active TB as necessary, even for patients who have completed preventive treatmentirrespective of TPT offer.
- 45. TREATMENTOFMDR-TB Goals of TB treatment •Render the patient non-infectious, break the chain of transmission and decrease pool of infection. •Decrease TB deaths and related comorbidity by ensuring relapse-free cure. •Minimize & prevent development and amplification of drug resistance.
- 47. Shorter oral Bedaquiline-containing MDR/RR-TB regimen - Shorter oral Bedaquiline-containing MDR/RR-TB regimen is recommended for those MDR/ RR-TB patients in whom resistance to the component drugs has been excluded or those who have not been previously treated for more than one month with second-line drugs used in shorter oral Bedaquiline- containing MDR/RR-TB regimen and have no other exclusion criteria. -The regimen consists of an initial phase of 4 months that may be extended up to 6 months and a continuation phase of 5 months, giving a total duration of 9–11 months. Bdq is used for a duration of 6 months. (4-6) Bdq (6 m), Lfx, Cfz, Z, E, Hh, Eto. (5) Lfx, Cfz, Z, E, Inclusion criteria ►► Rifampicin resistance detected/inferred►► MDR/RR-TB with H resistance detected/inferred based on InhA mutation only or based on KatG mutation only (not both) ►► MDR/RR-TB with FQ resistance not detected Exclusion criteria ►► MDR/RR-TB patients with H resistance detected with both KatG and InhA mutation; and ►► MDR/RR-TB patients with FQ resistance detected.
- 49. Duration:- Longeroral M/XDR-TB regimen is of 18-20 months with no separate IP or CP. Although standardization in the designof longerregimens is possible,in many cases,the modificationof the compositionand duration of a regimento make it individualized could enhance regimen effectiveness orsafety(or both). Longer oral M/XDR-TB regimen Eligibility criteria:- • Longeroral M/XDR-TB regimen is recommendedforMDR/RR-TB patients who are excluded from shorter oral Bedaquiline-containing MDR/RR-TB regimenincluding for the XDRTB patients. • In case of additional resistance or intolerance or non-availability of any drug in use or emergence of exclusion criteria to shorter oral Bedaquiline-containing MDR/RR-TB regimenor any longer regimen,the patient would be re-evaluated and initiated on longer oral M/XDR-TB regimenat N/DDR-TBC with any modifications,if additional resistance to any second-line drugs especially Lfx, Mfx, Bdq*,Lzd, Cfz*, Dlm* and Z (*whenever available) 18-20)Lfx Bdq (6 month or longer) Lzd Cfz Cs
- 51. Side effects of commonly used drugs in MDR TB
- 52. Adverse Drug reaction :
- 53. Lab investigation to monitor ADR
- 54. OnceTPT is initiated, the individuals will be monitored by the Doctor for clinical and laboratory parameters as below:- ● Screening with 4S symptoms (cough, fever,night sweats and weight loss) ● Any side effects ● If any of the sign/ symptoms of TB emerge,the personmay be referred to the DR-TB centre forfurther evaluation foractive TB/DR-TB disease Adherenceto the TPT course and treatment completionare important determinants of clinical benefit, bothat individual and populationlevels.Irregular or inadequate treatment reduces the protective efficacyof TPTregimen.Pooradherence or early cessationof TPT can potentially increase the risk of the individual developing TB including drug-resistantTB