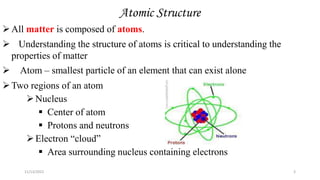
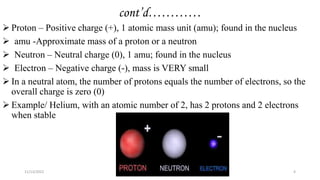
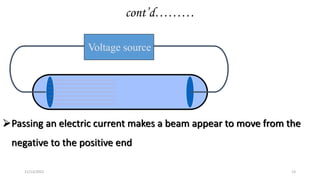
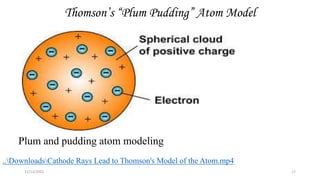
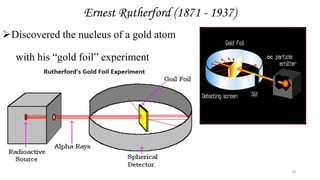
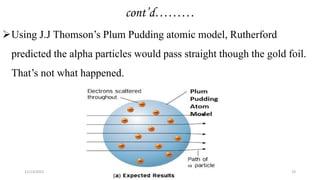
This document provides an overview of atomic structure and theory. It begins by discussing how an understanding of atomic structure relates to materials properties. It then reviews the atomic models of Democritus, Dalton, Thomson, Rutherford, Bohr, and others. Key concepts covered include the nucleus, protons, neutrons, electrons, atomic number, mass number, and energy levels. The document also discusses atomic bonding mechanisms like ionic bonding, covalent bonding, and metallic bonding. Finally, it introduces topics like energy bands and the quantization of energy and light.