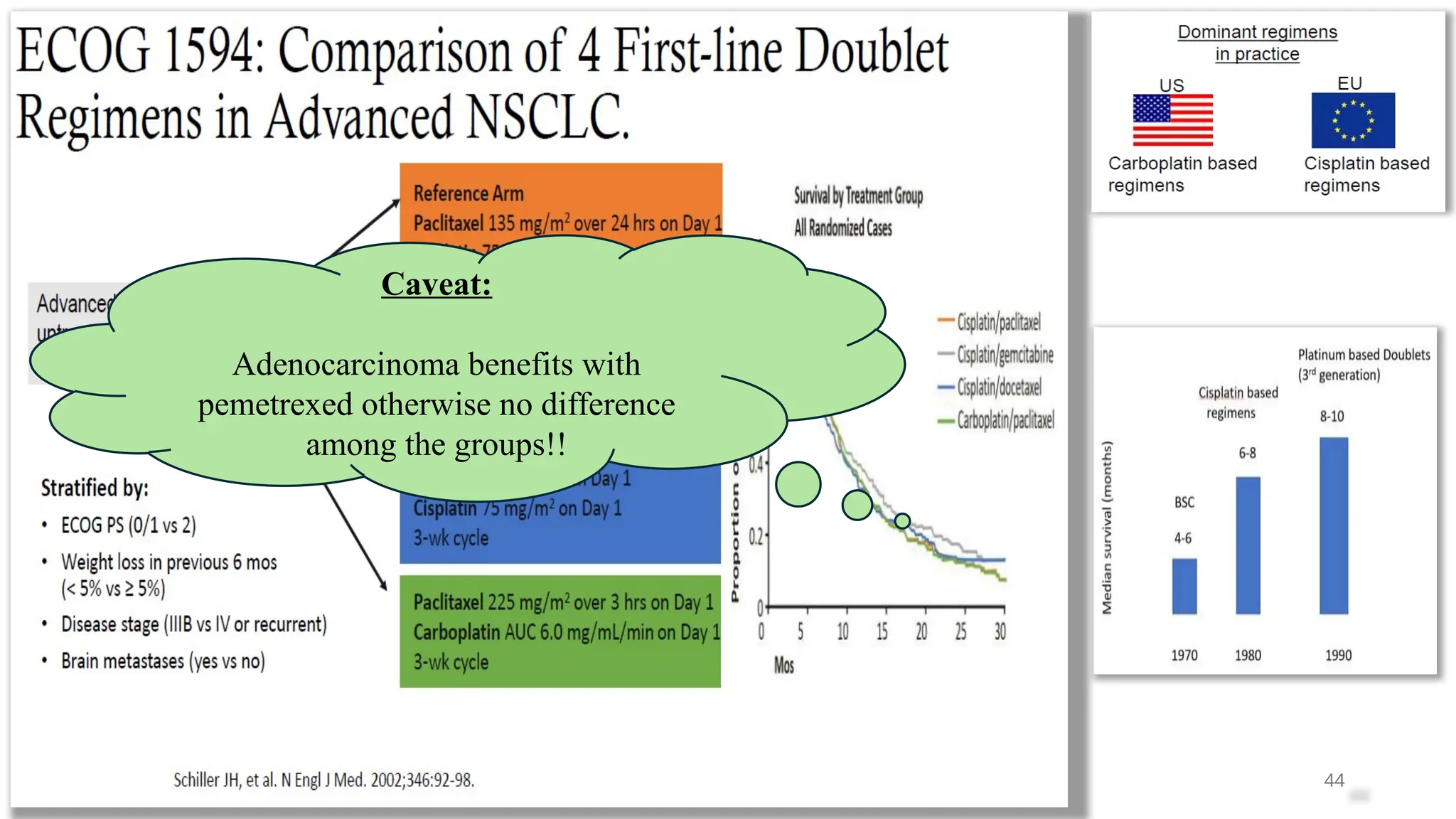
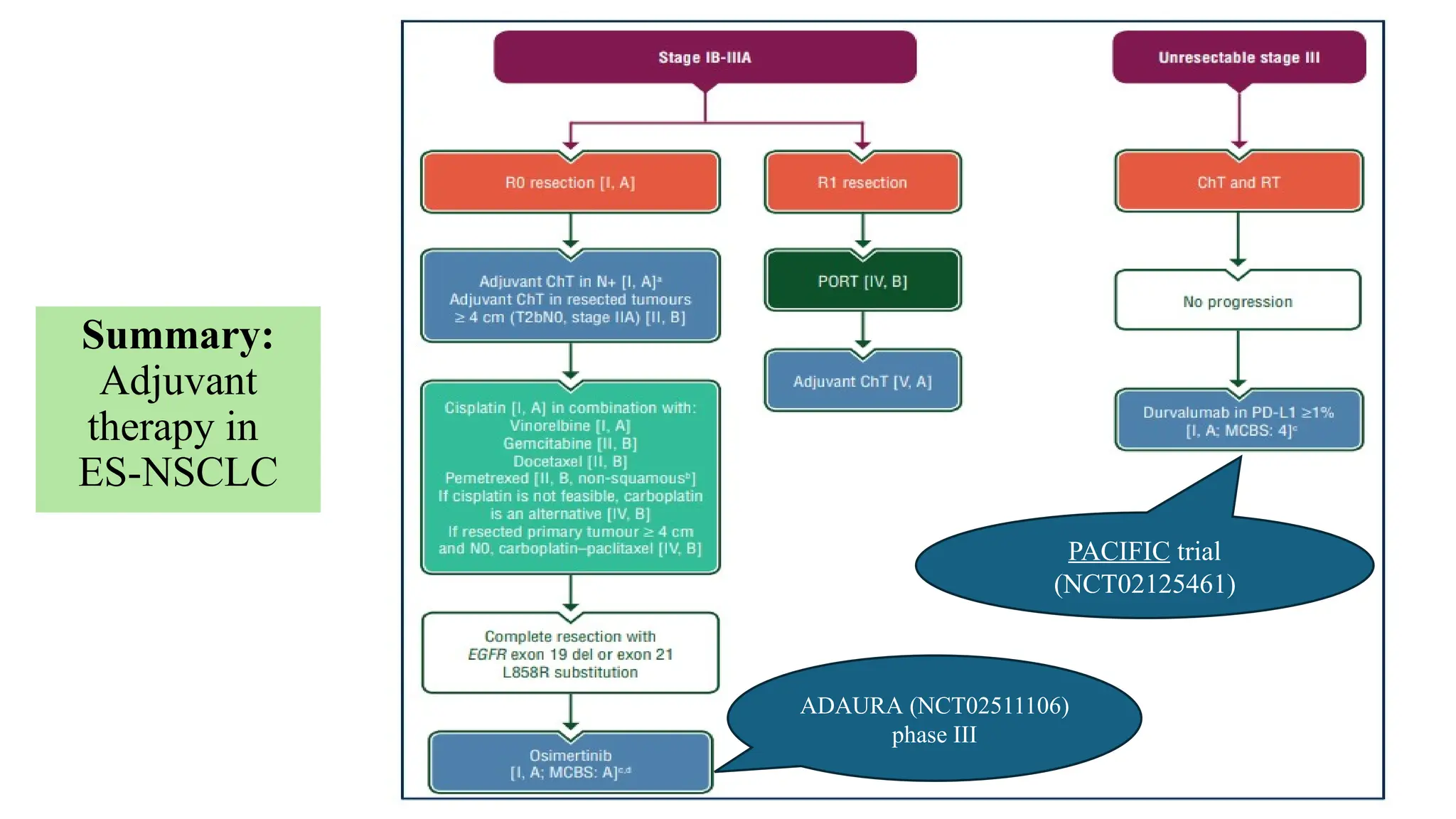
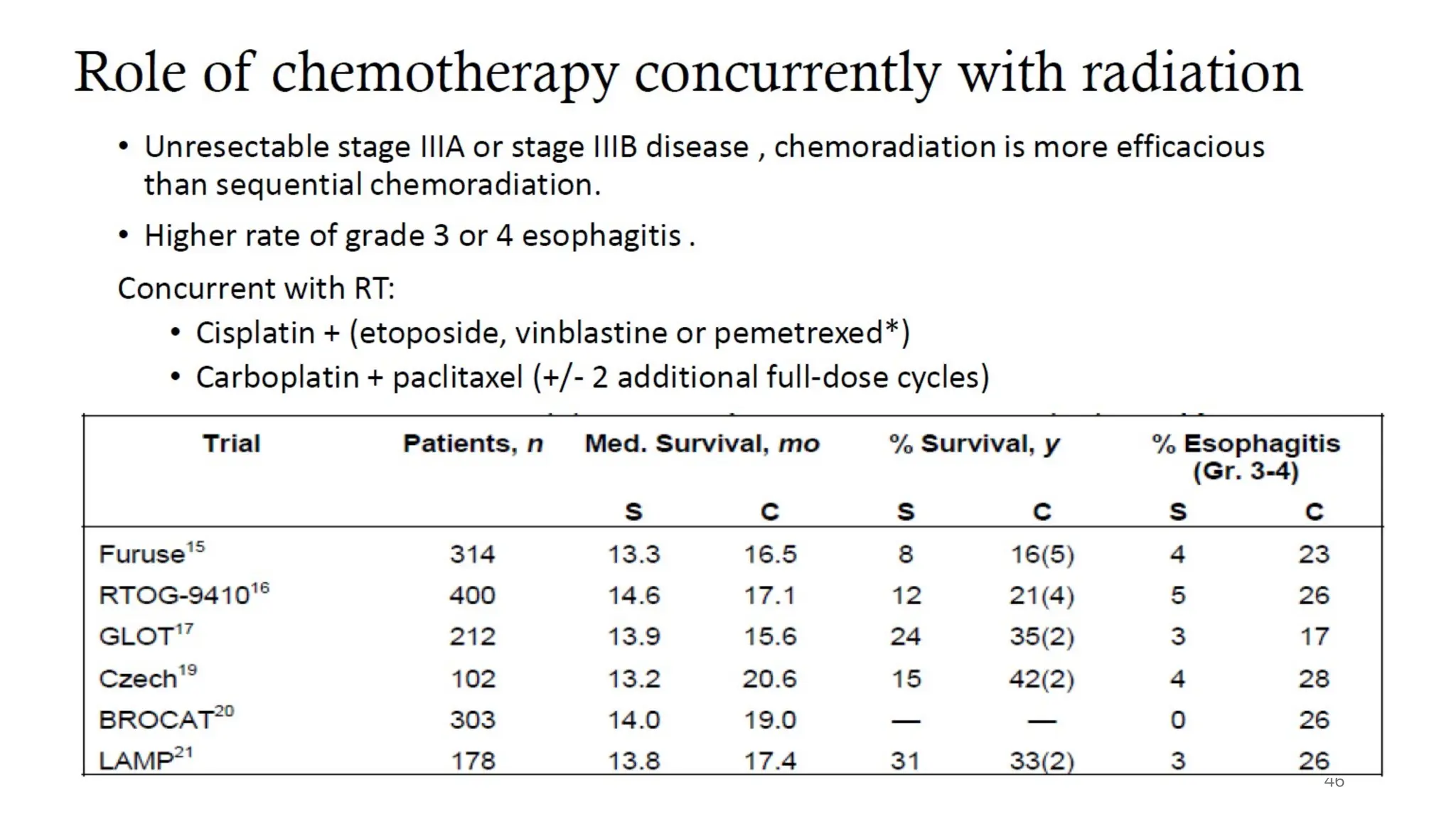
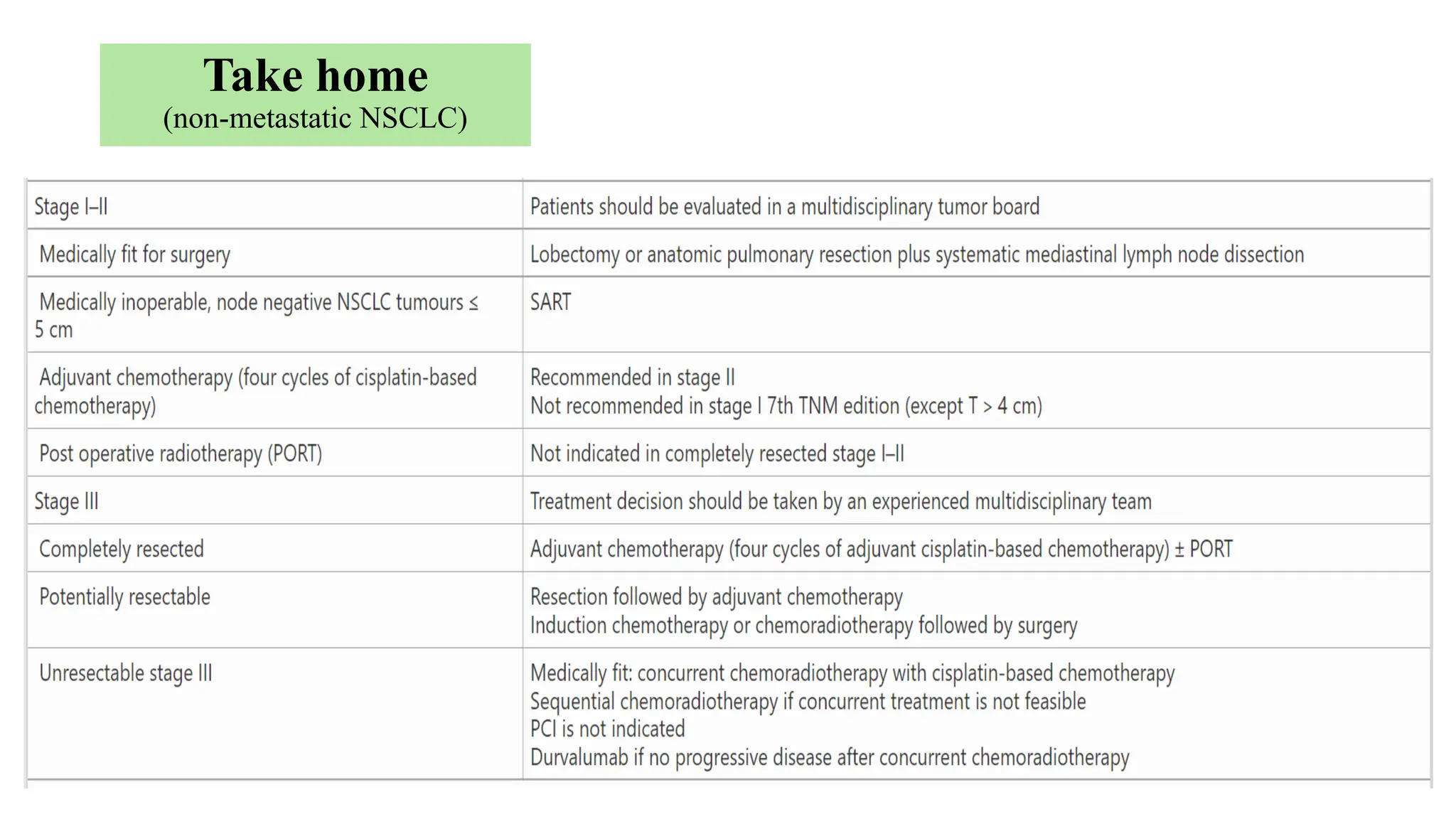
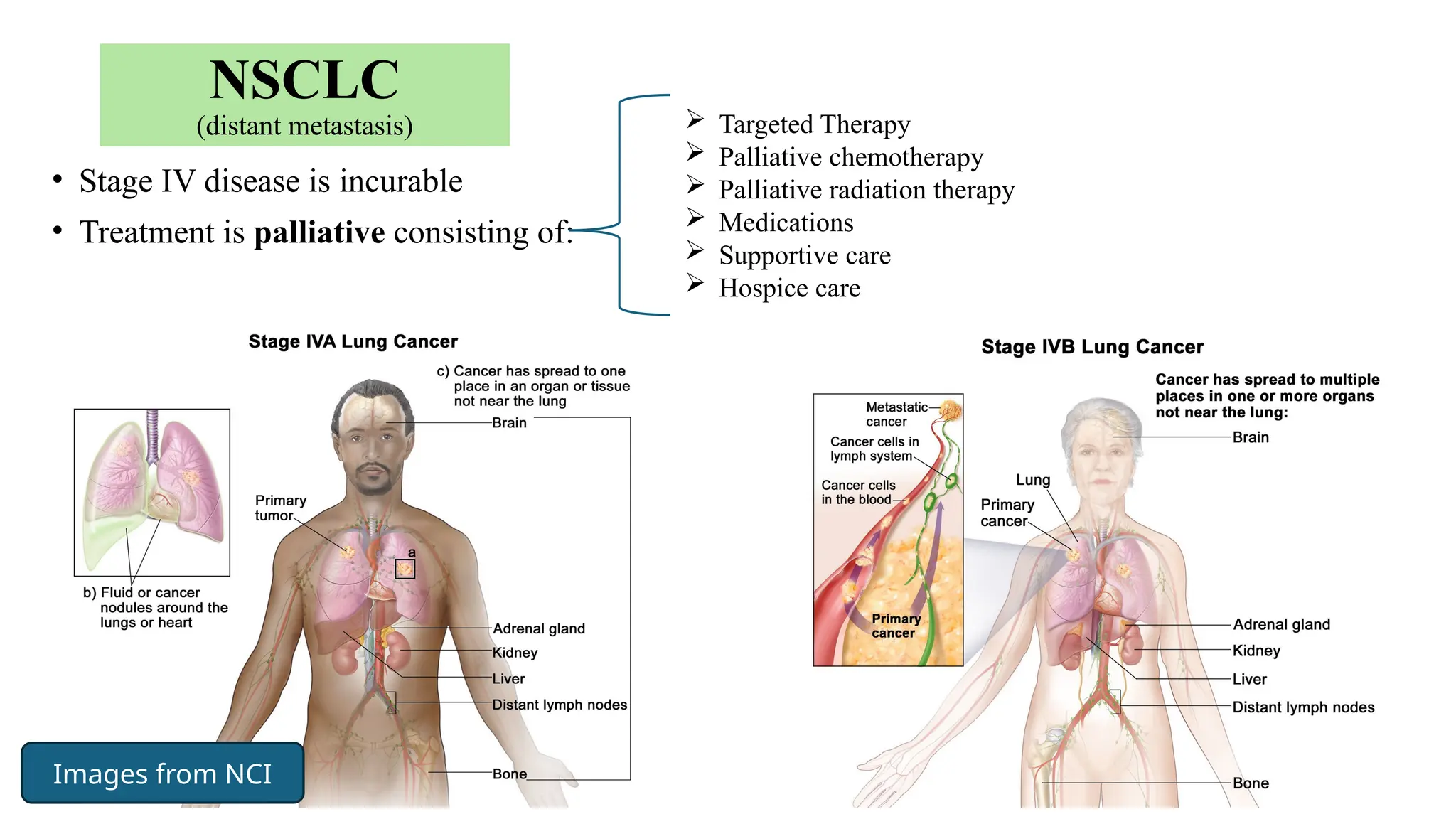
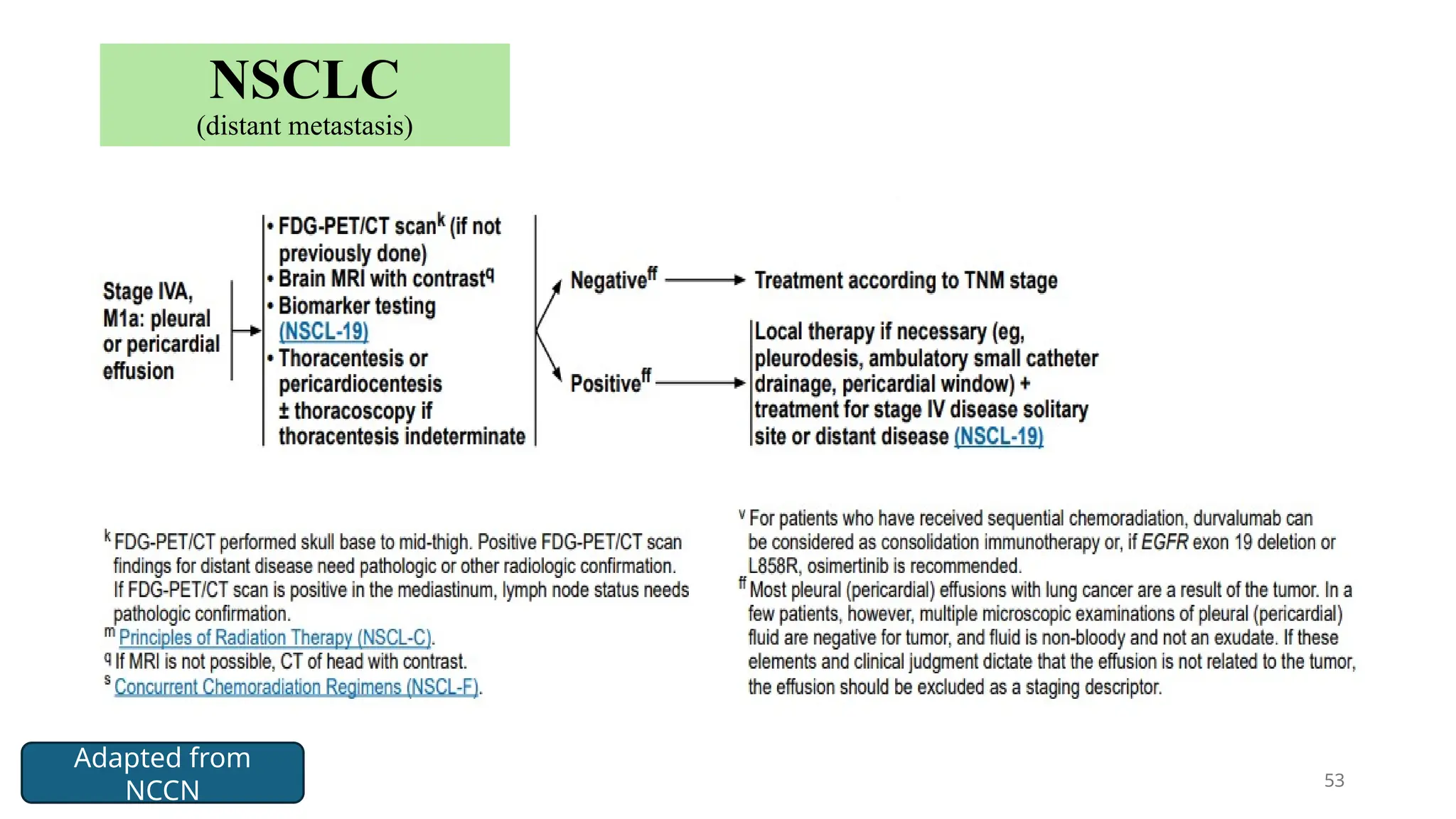
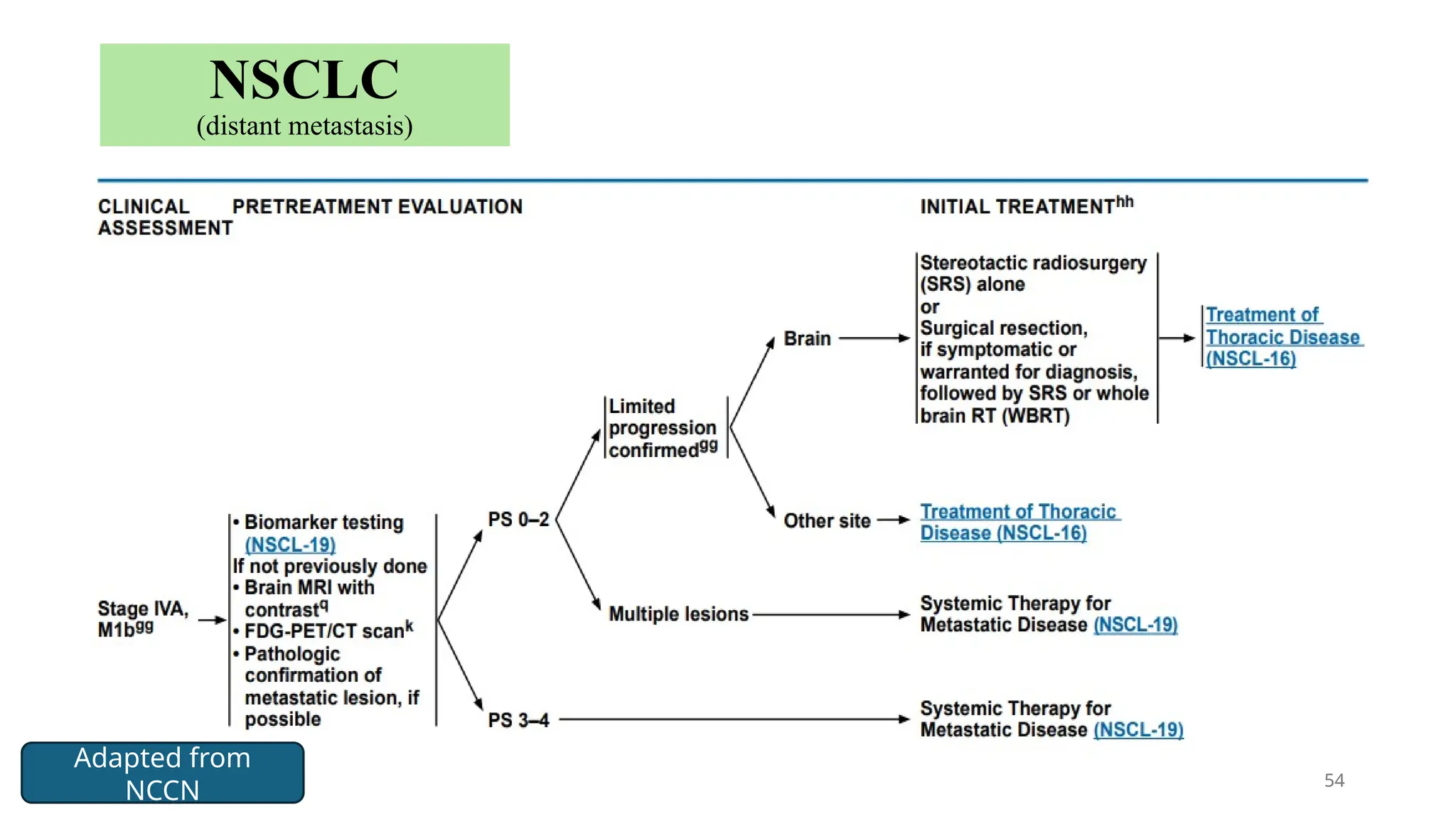
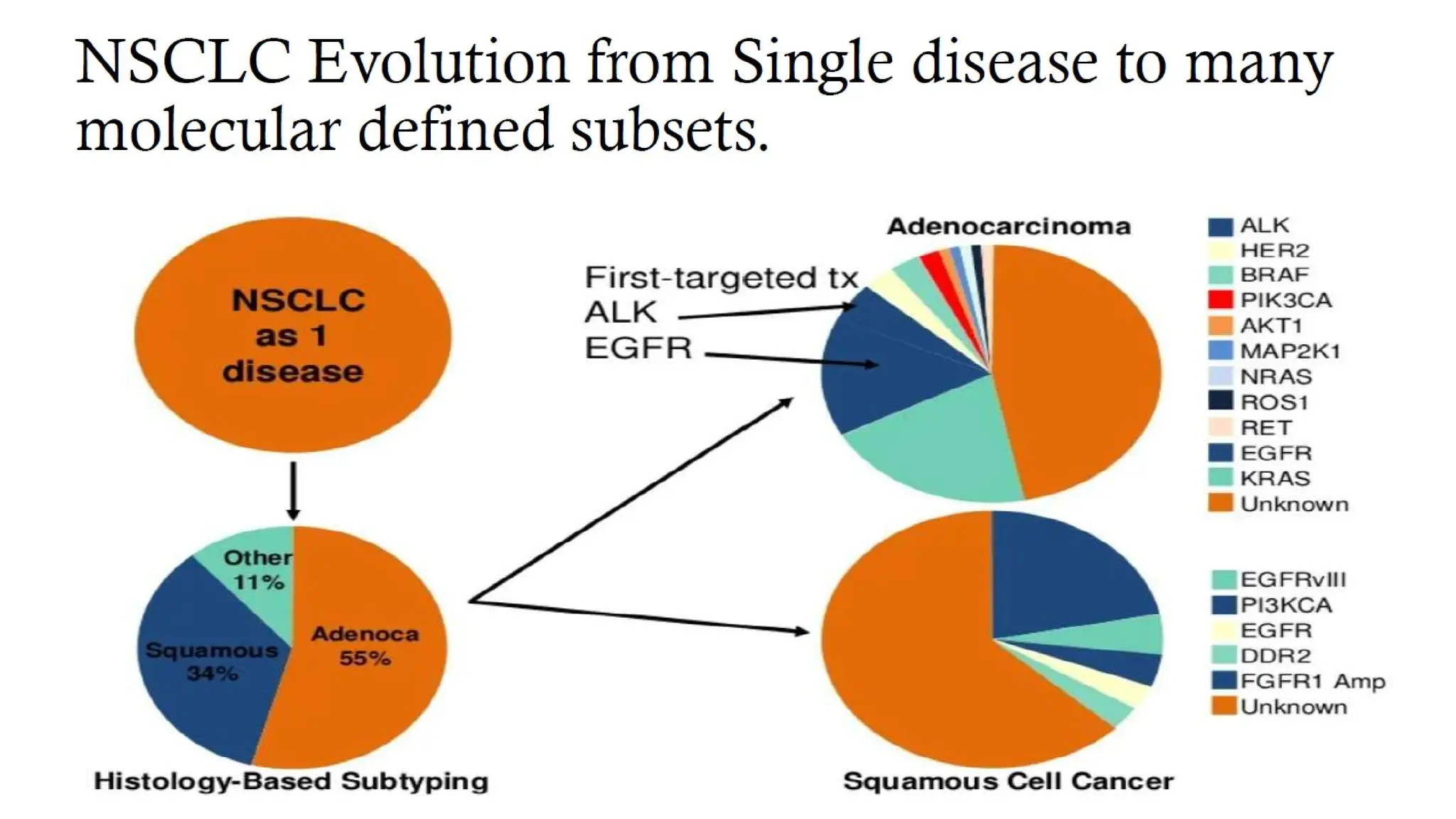
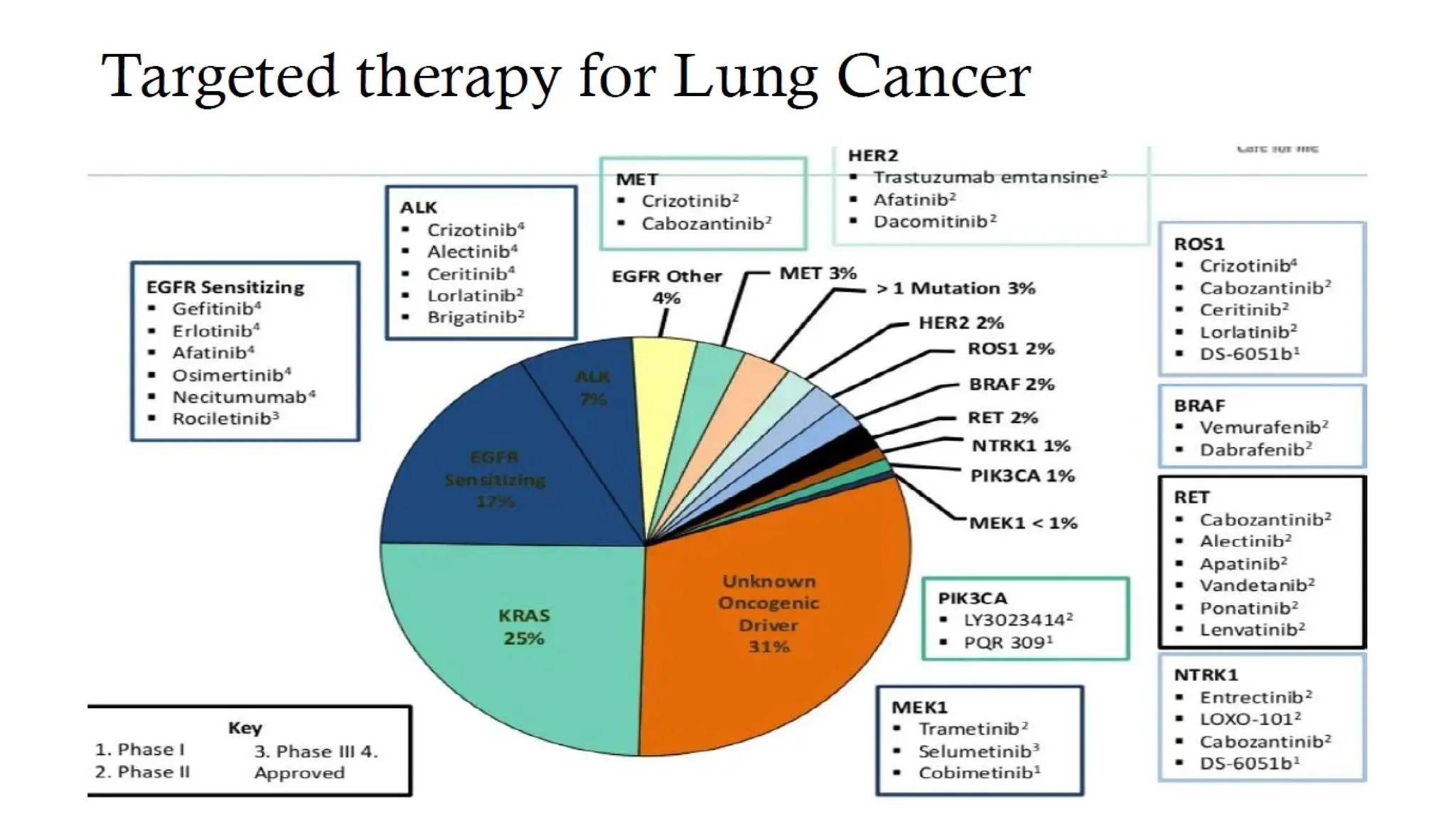
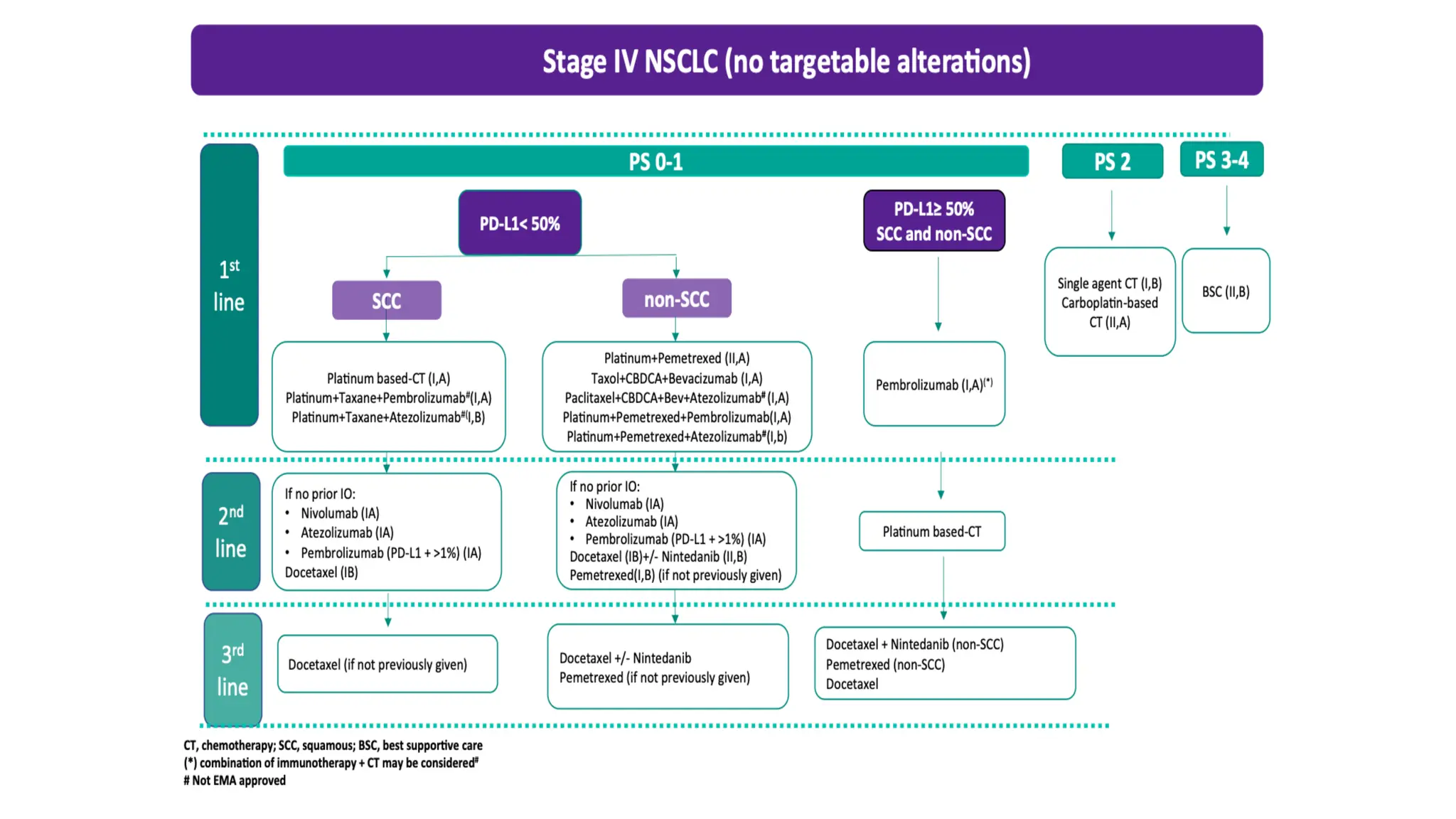
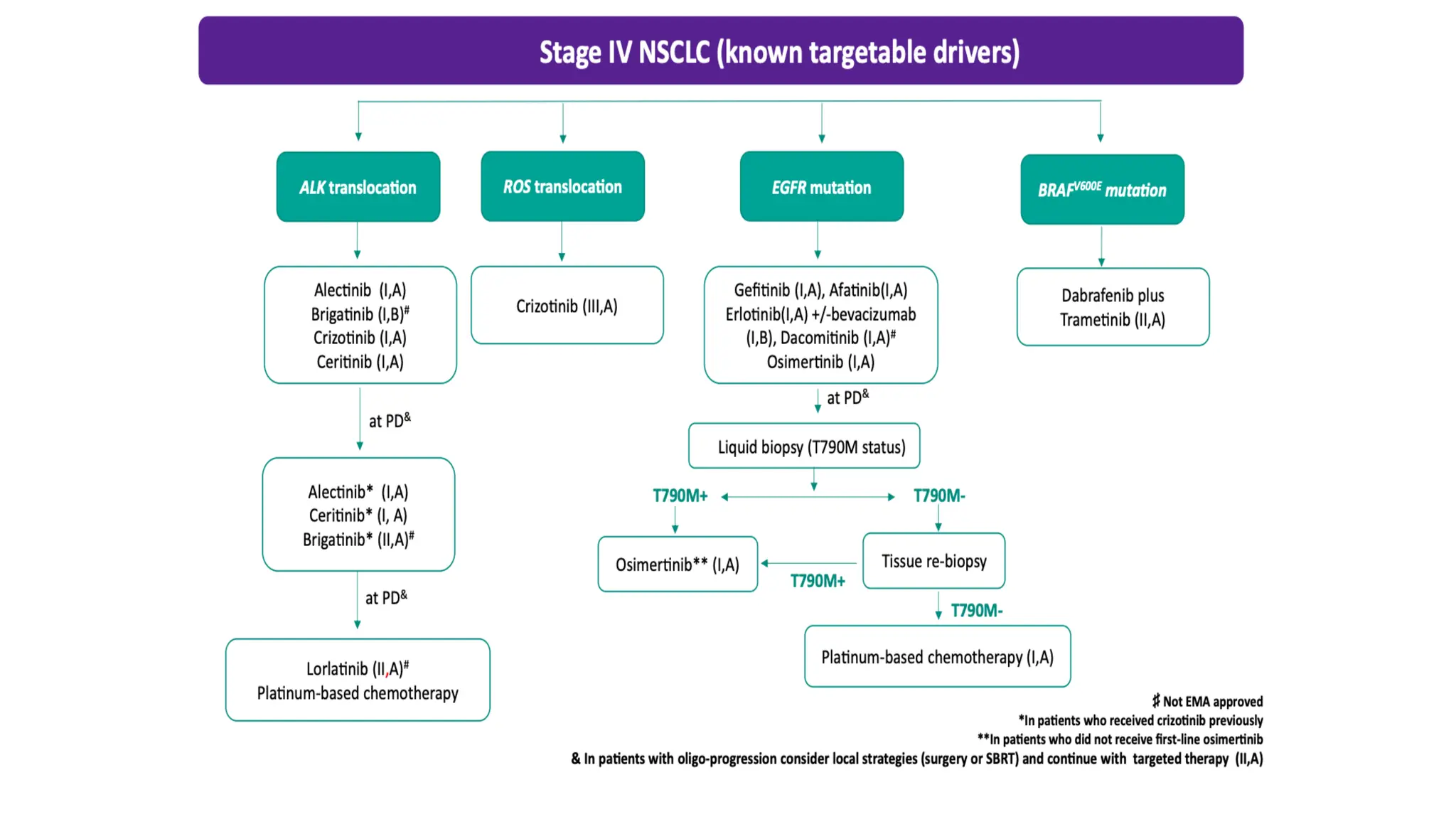
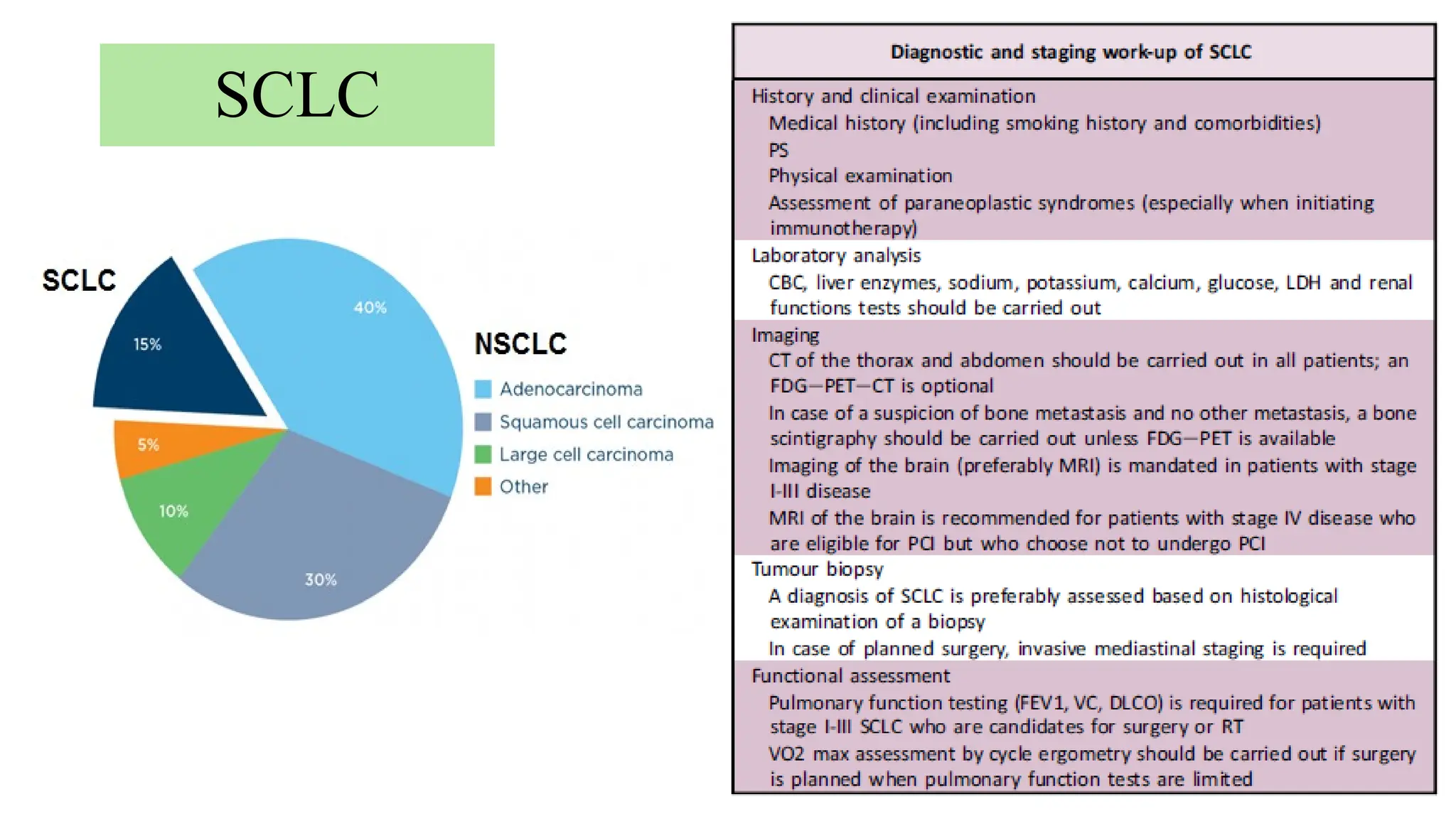
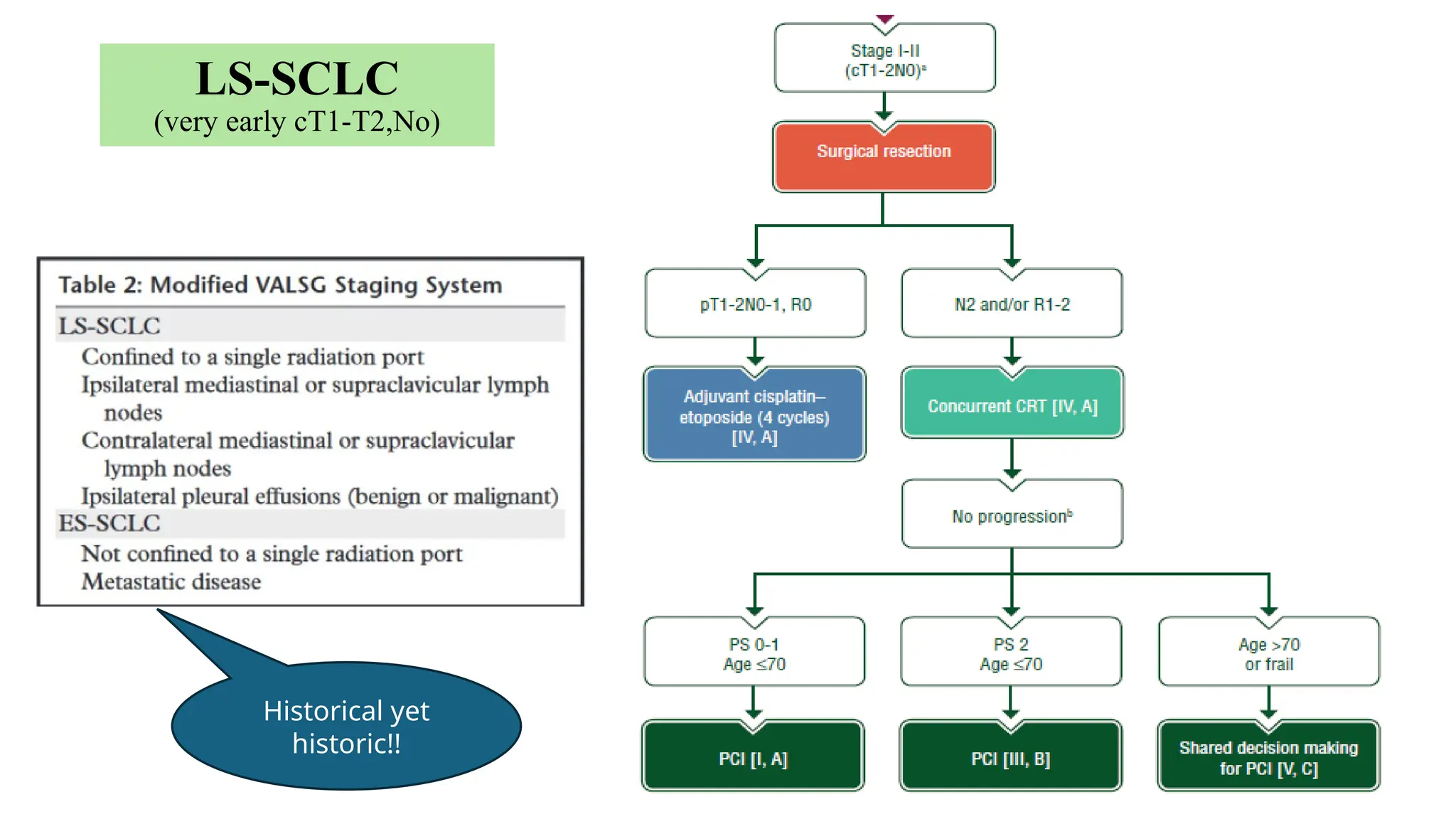
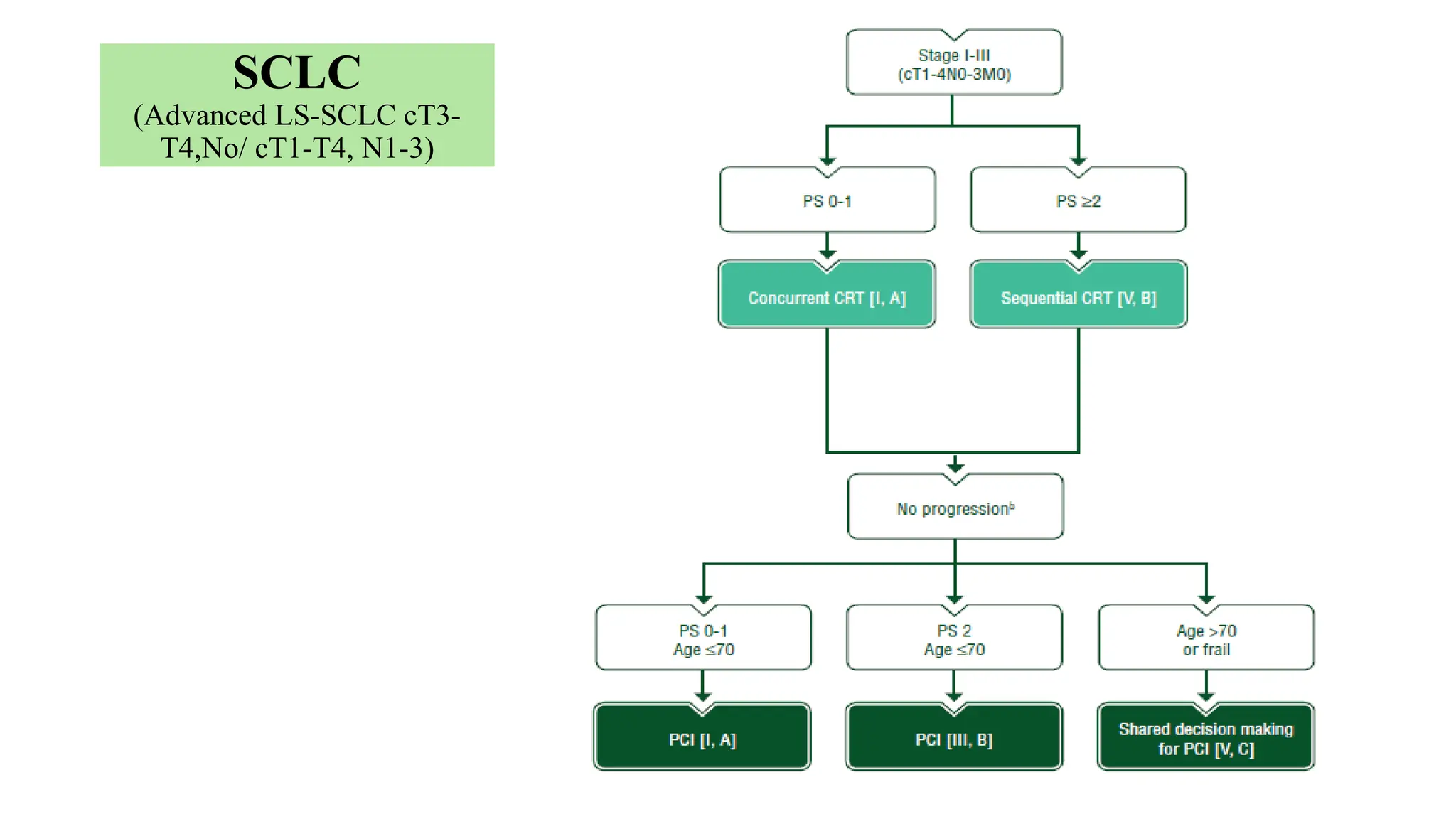
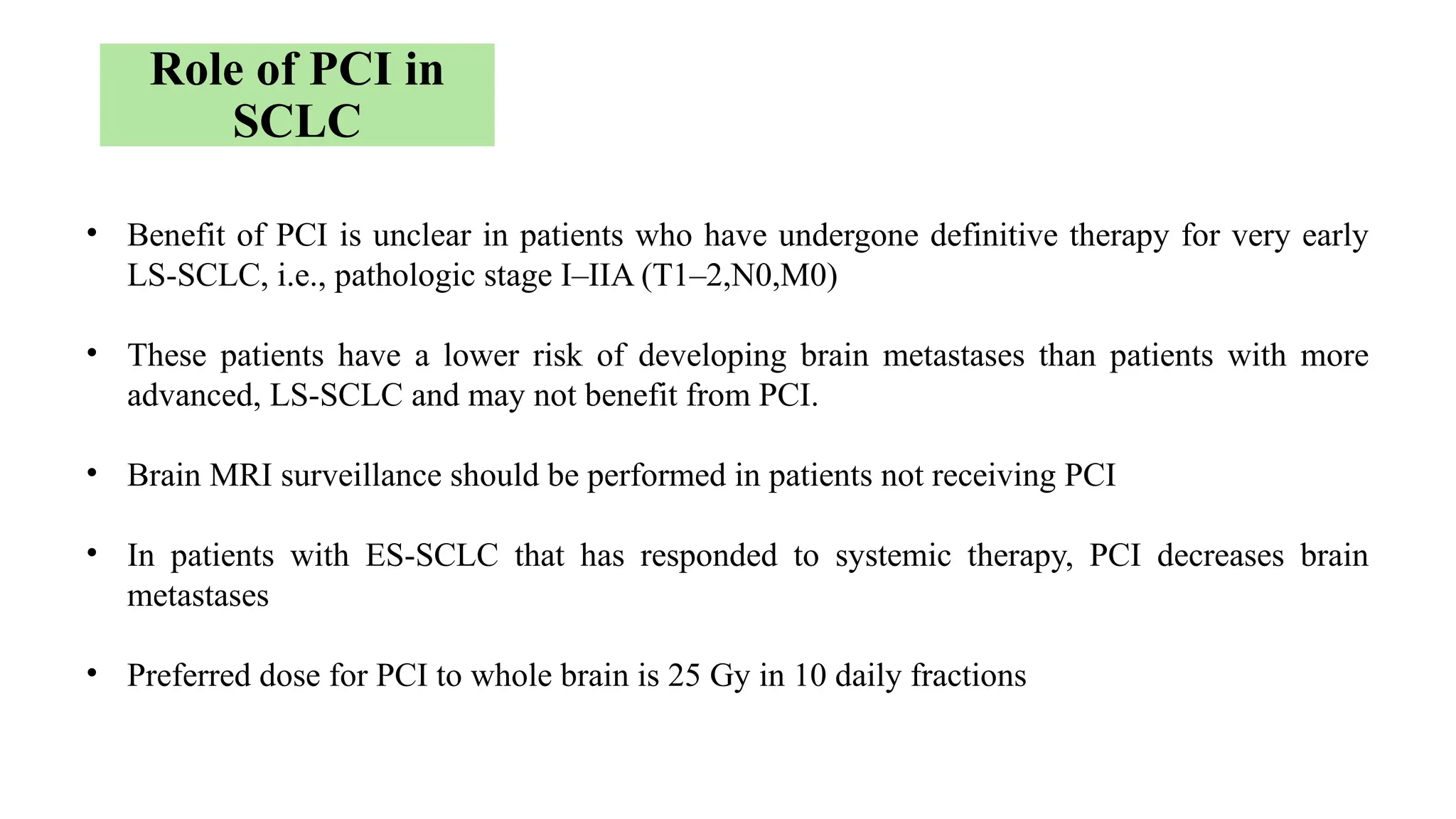
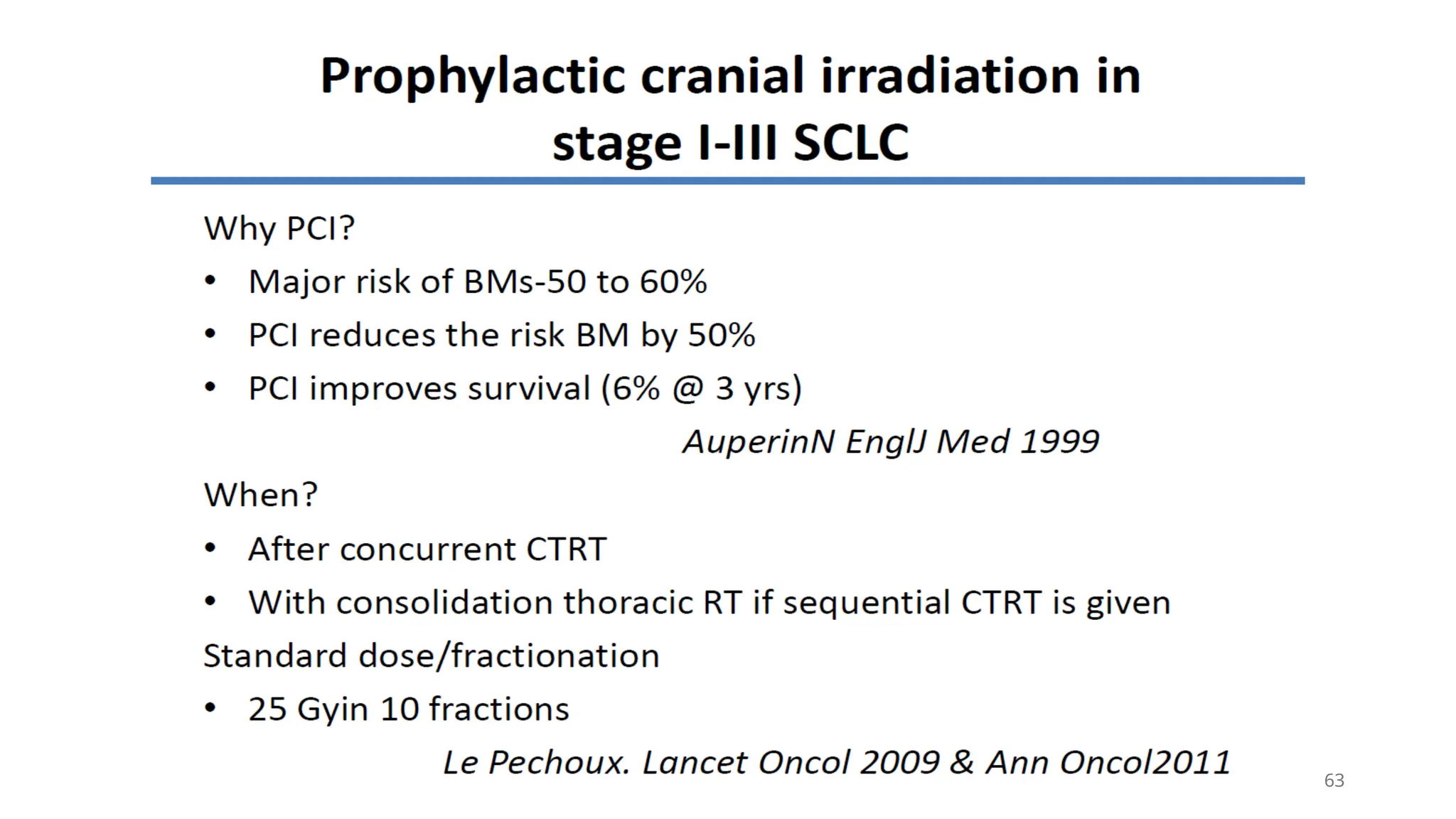
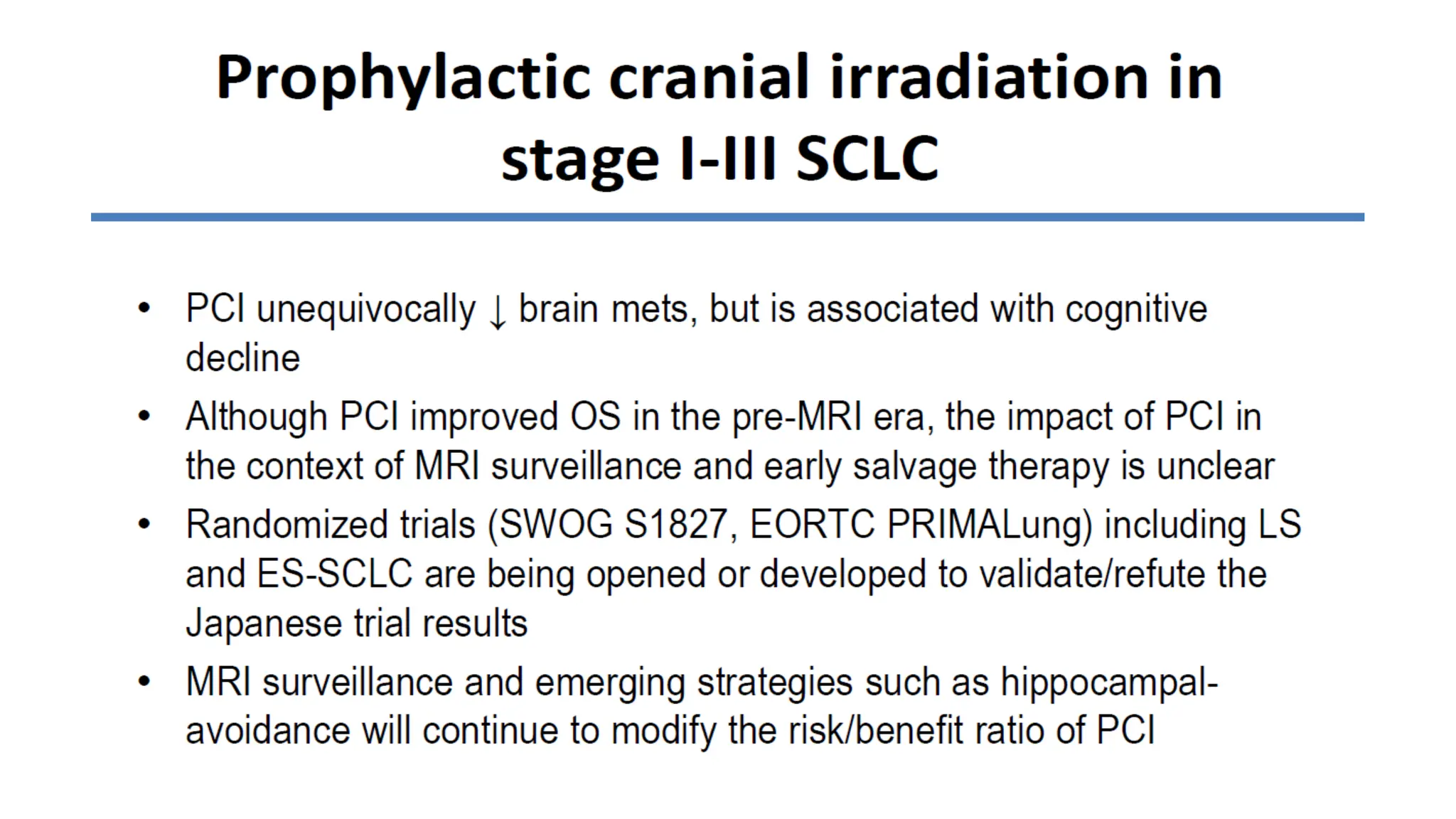
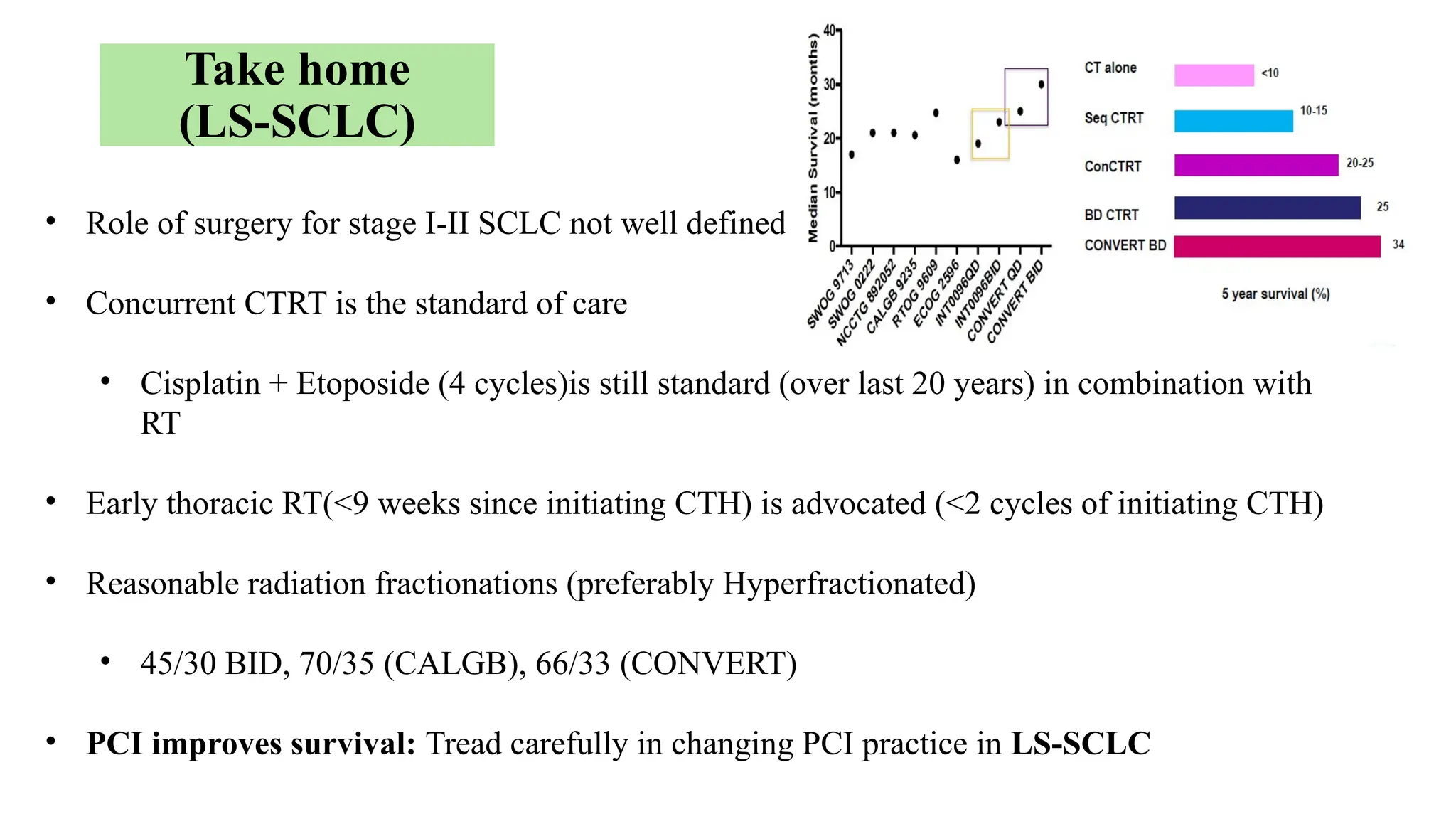
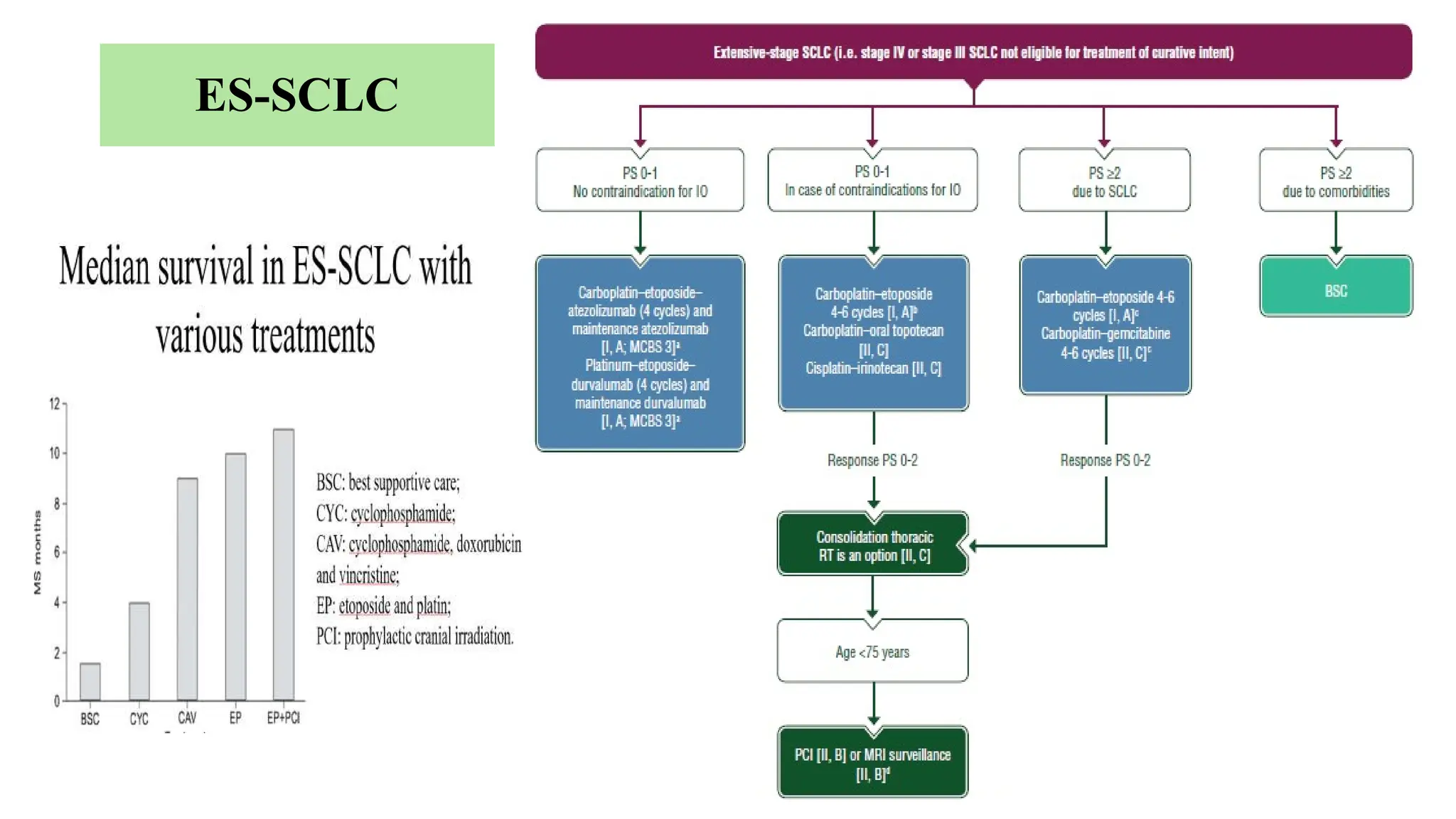
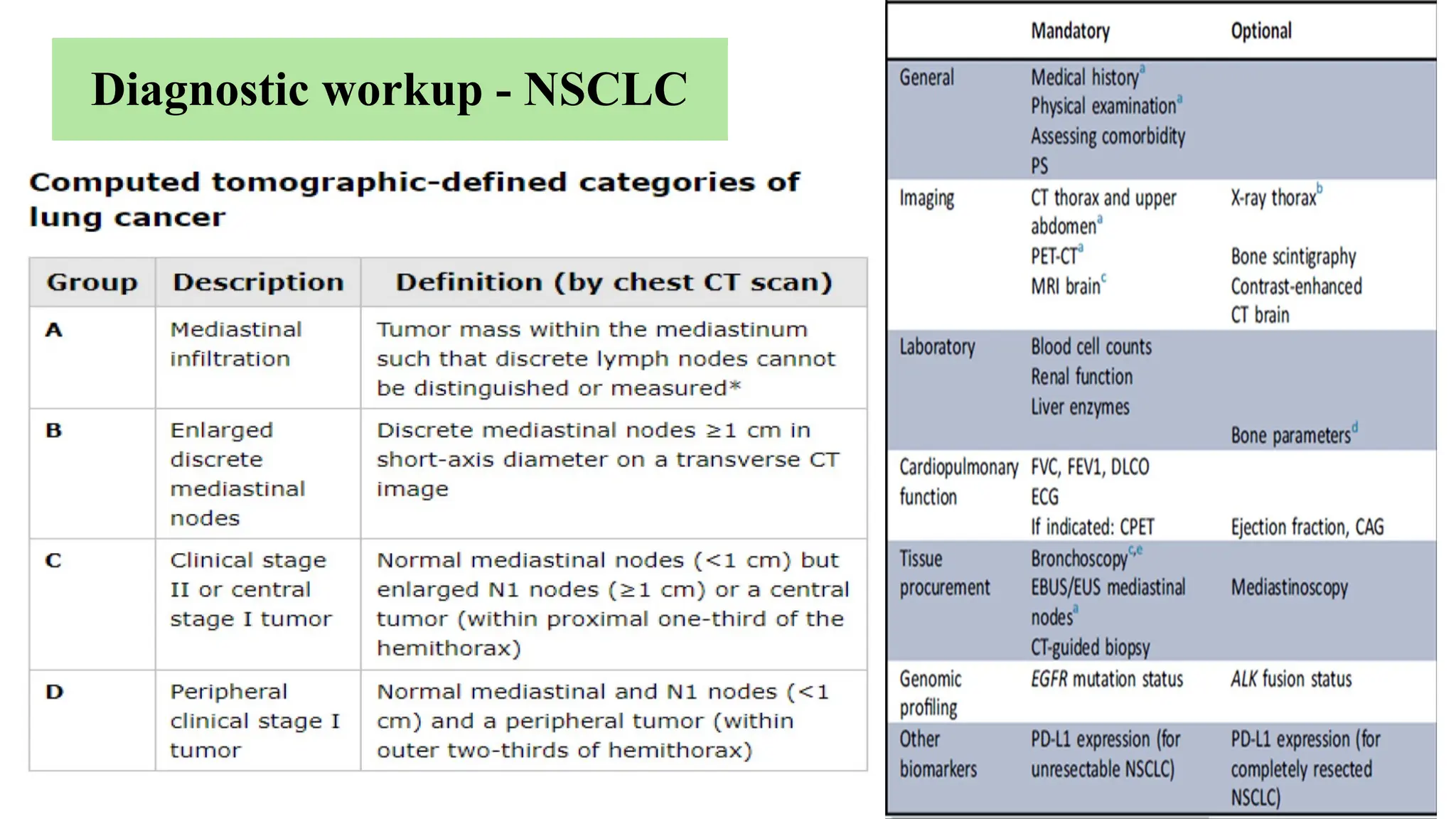
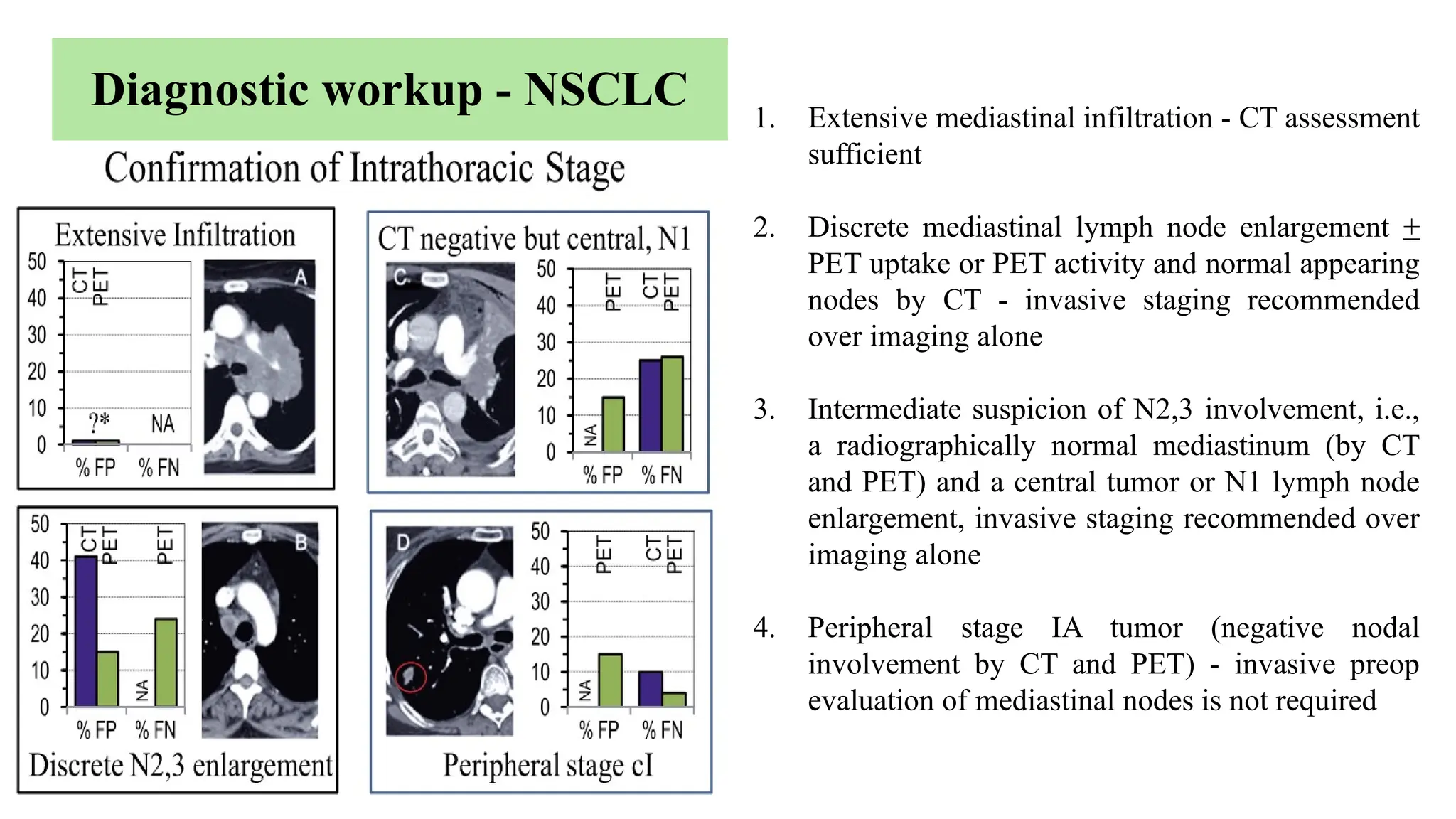
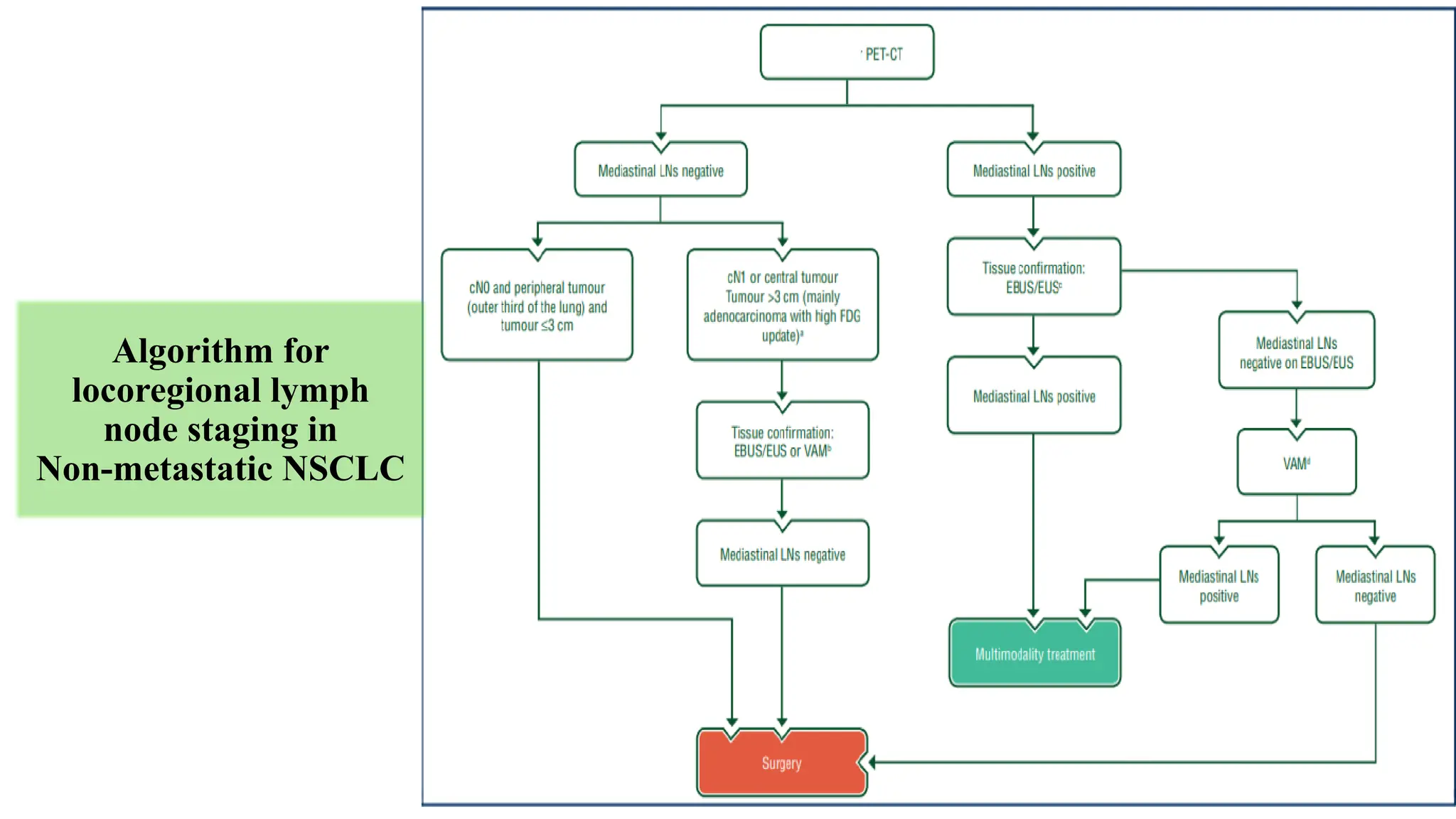
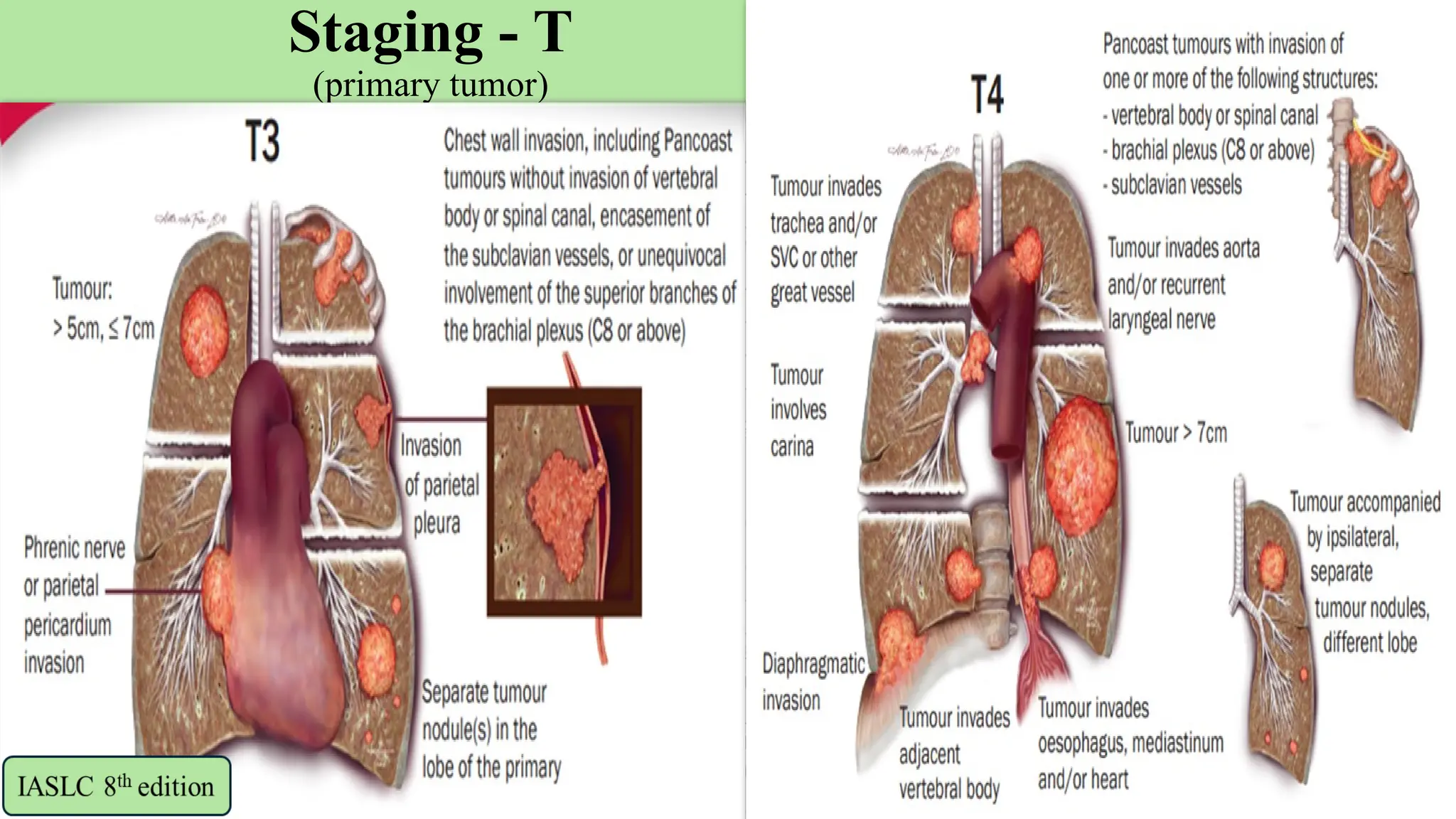
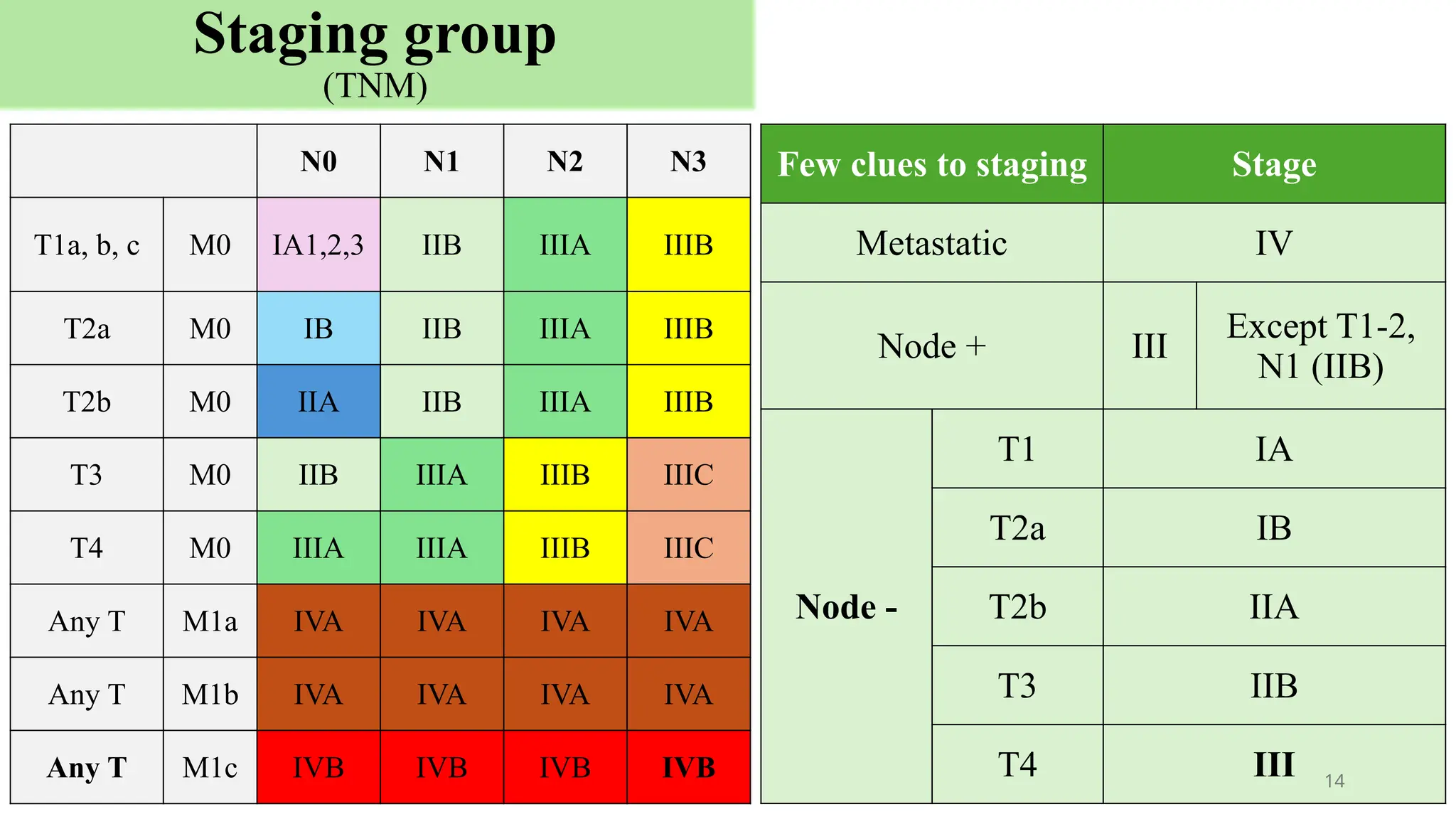
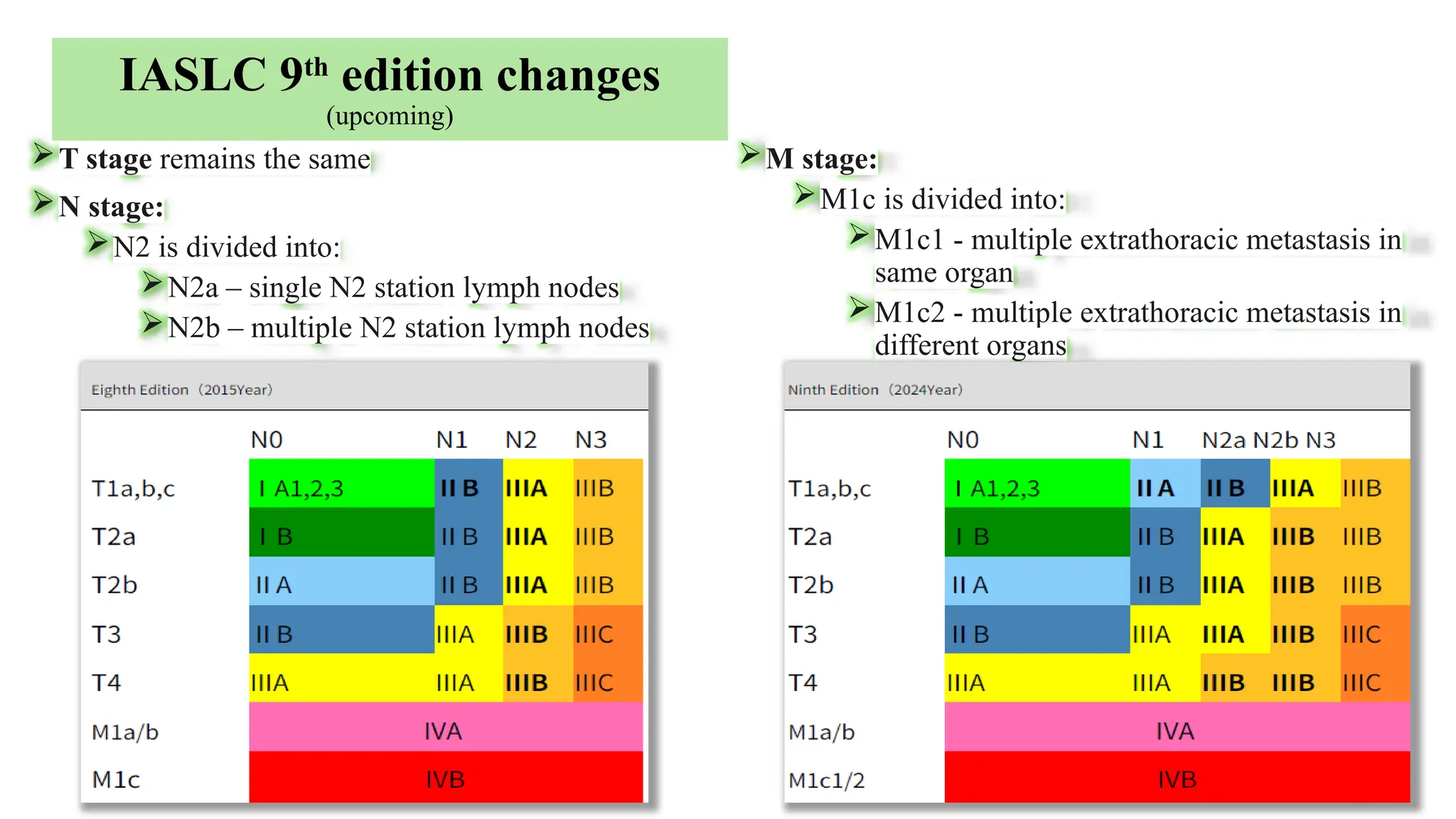
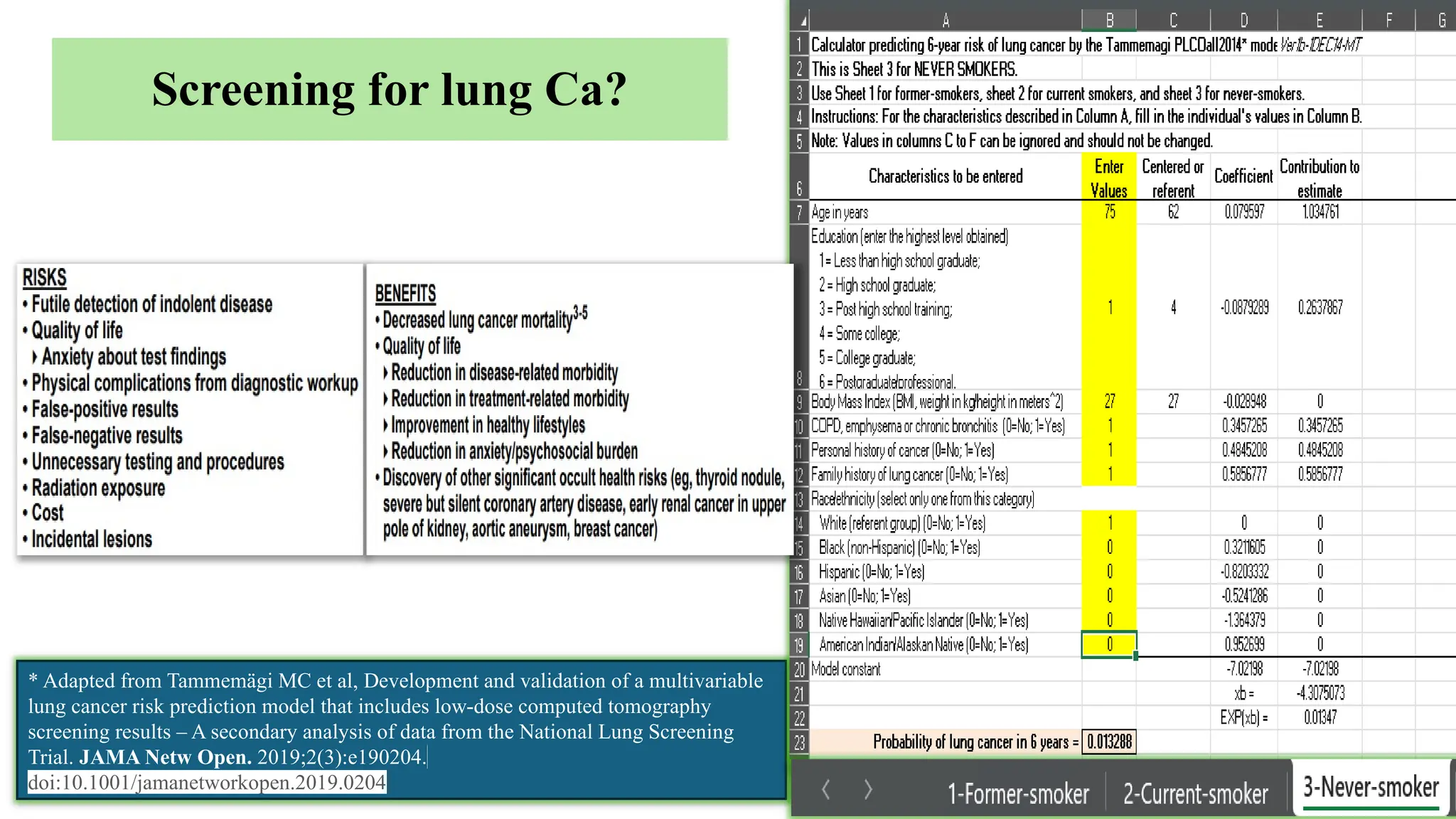
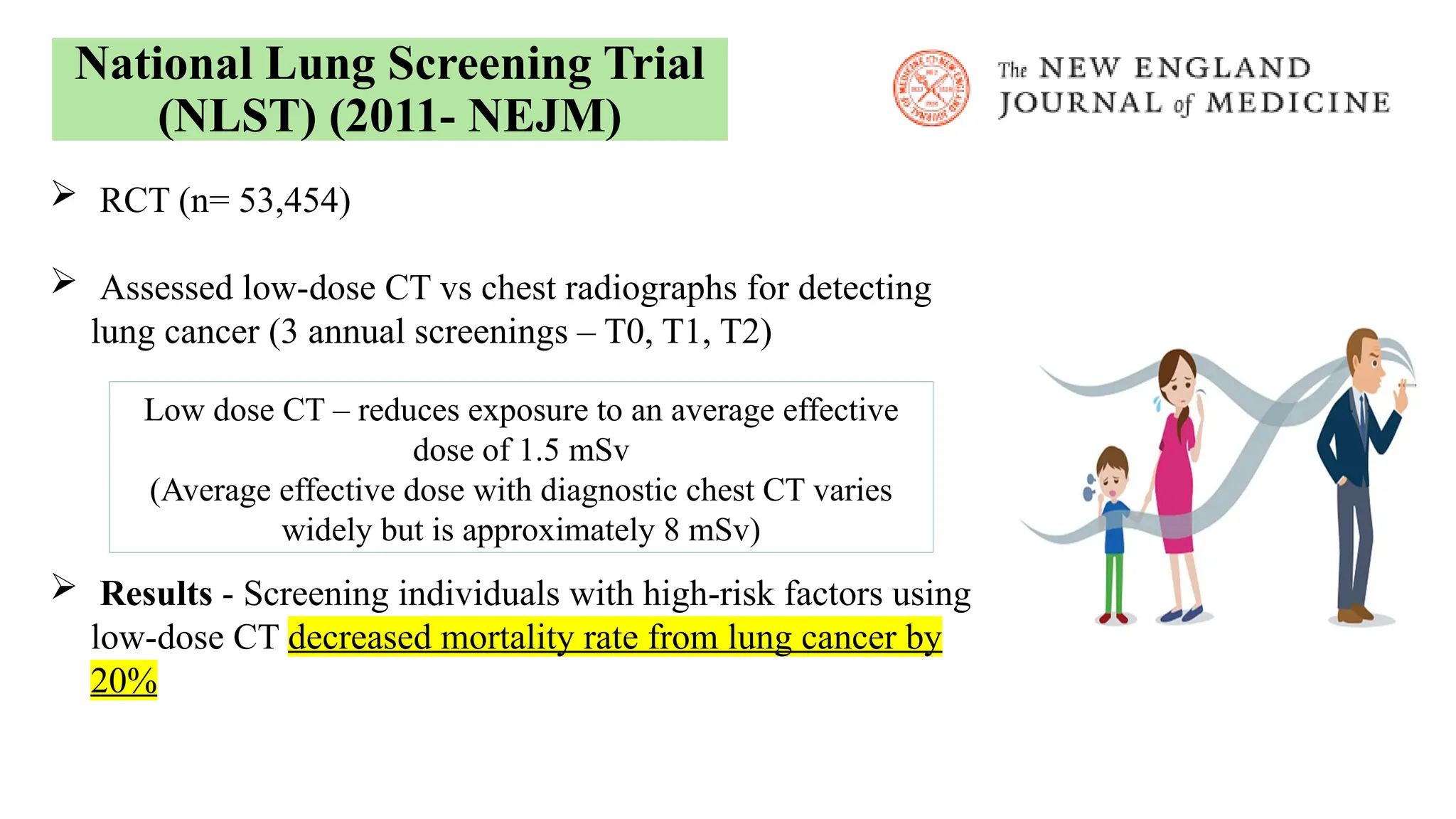
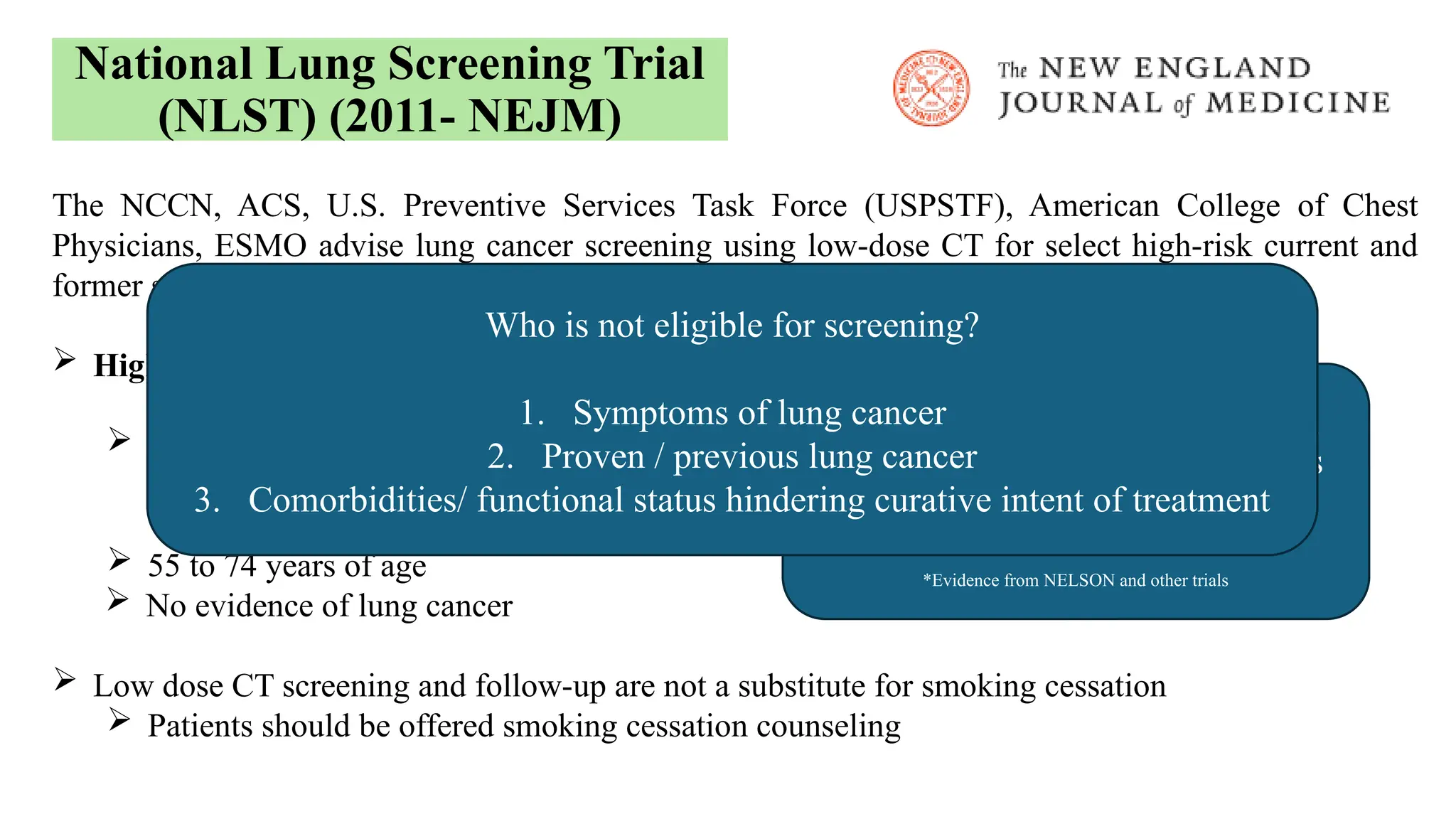
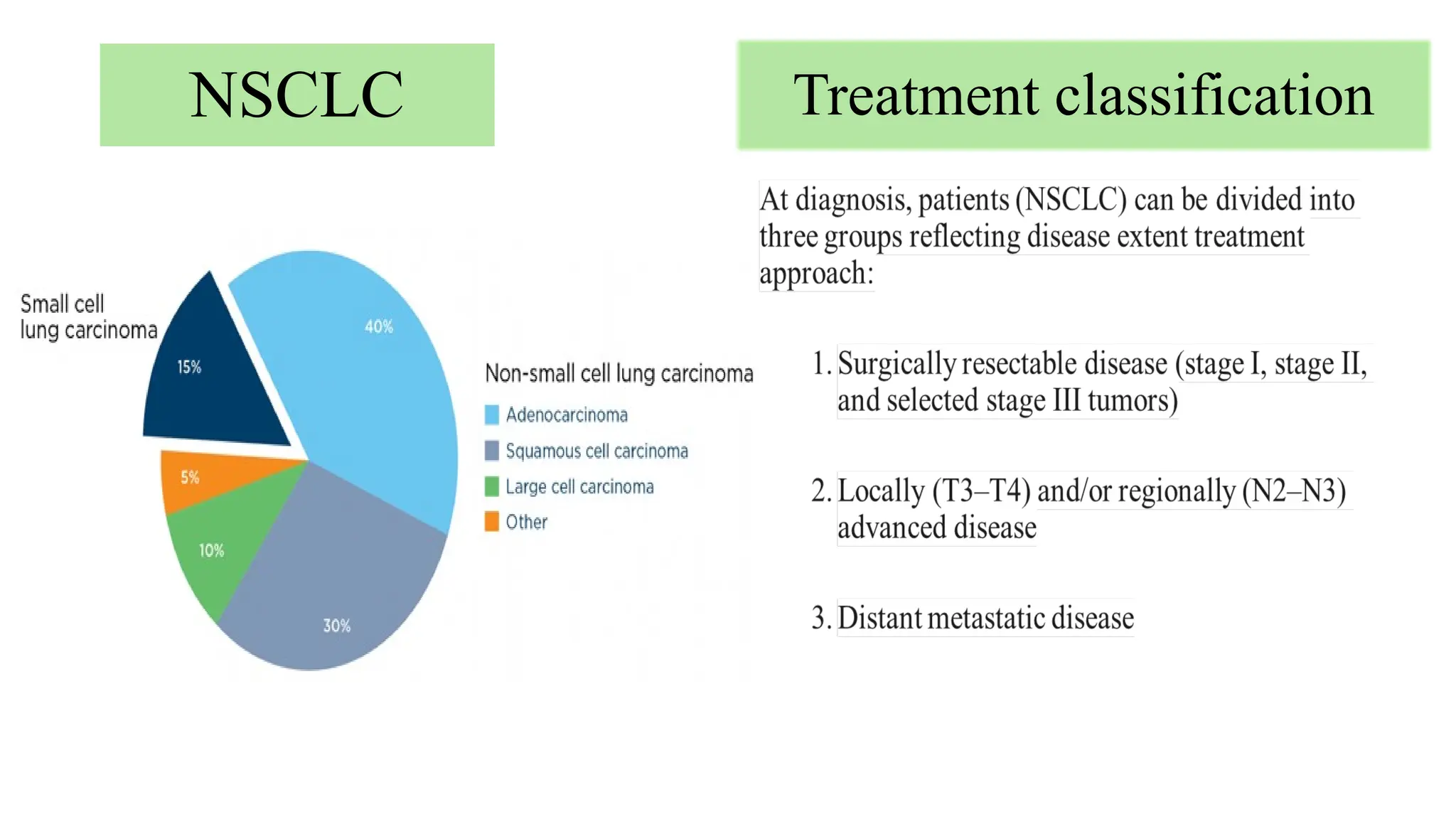
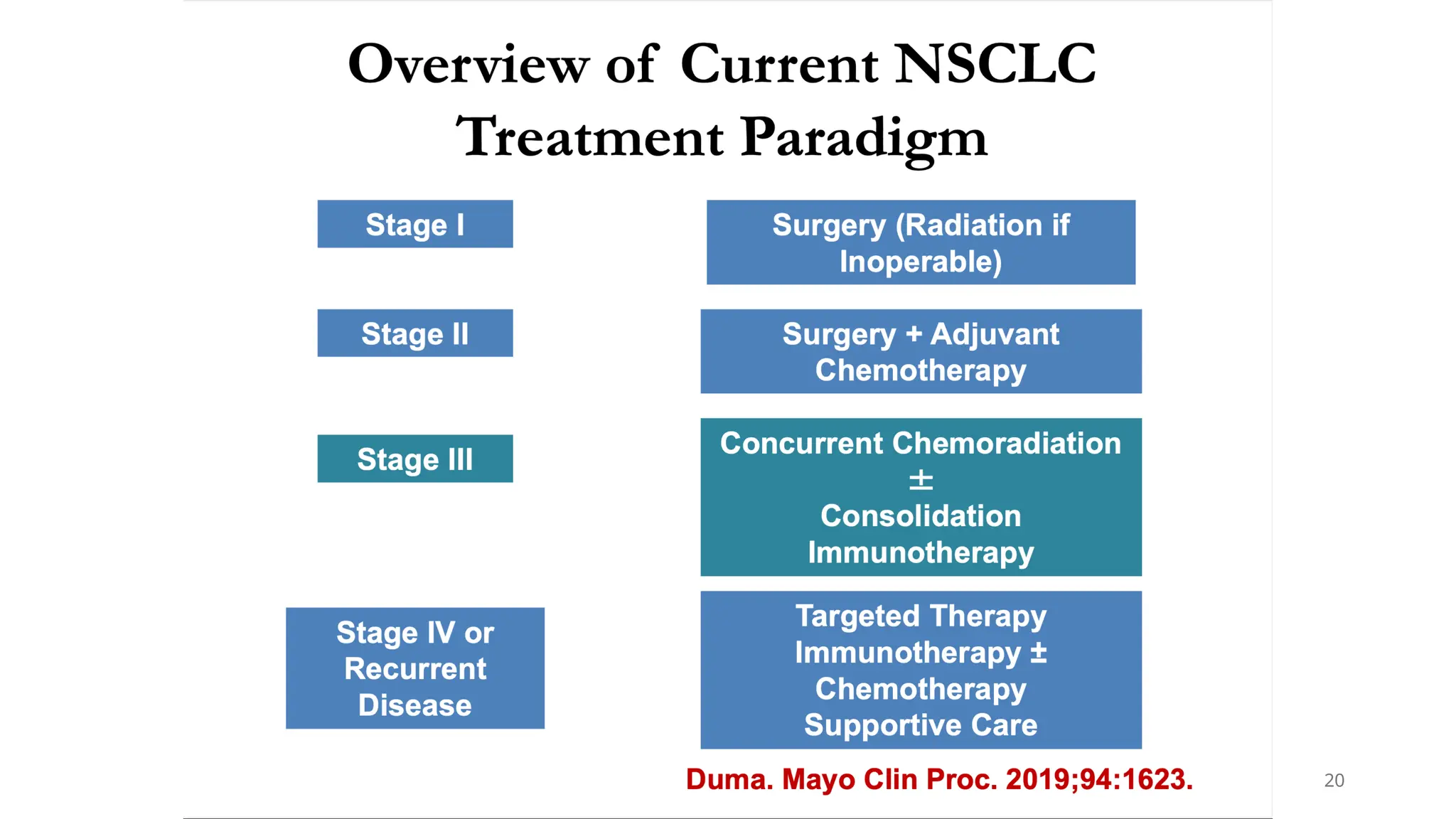
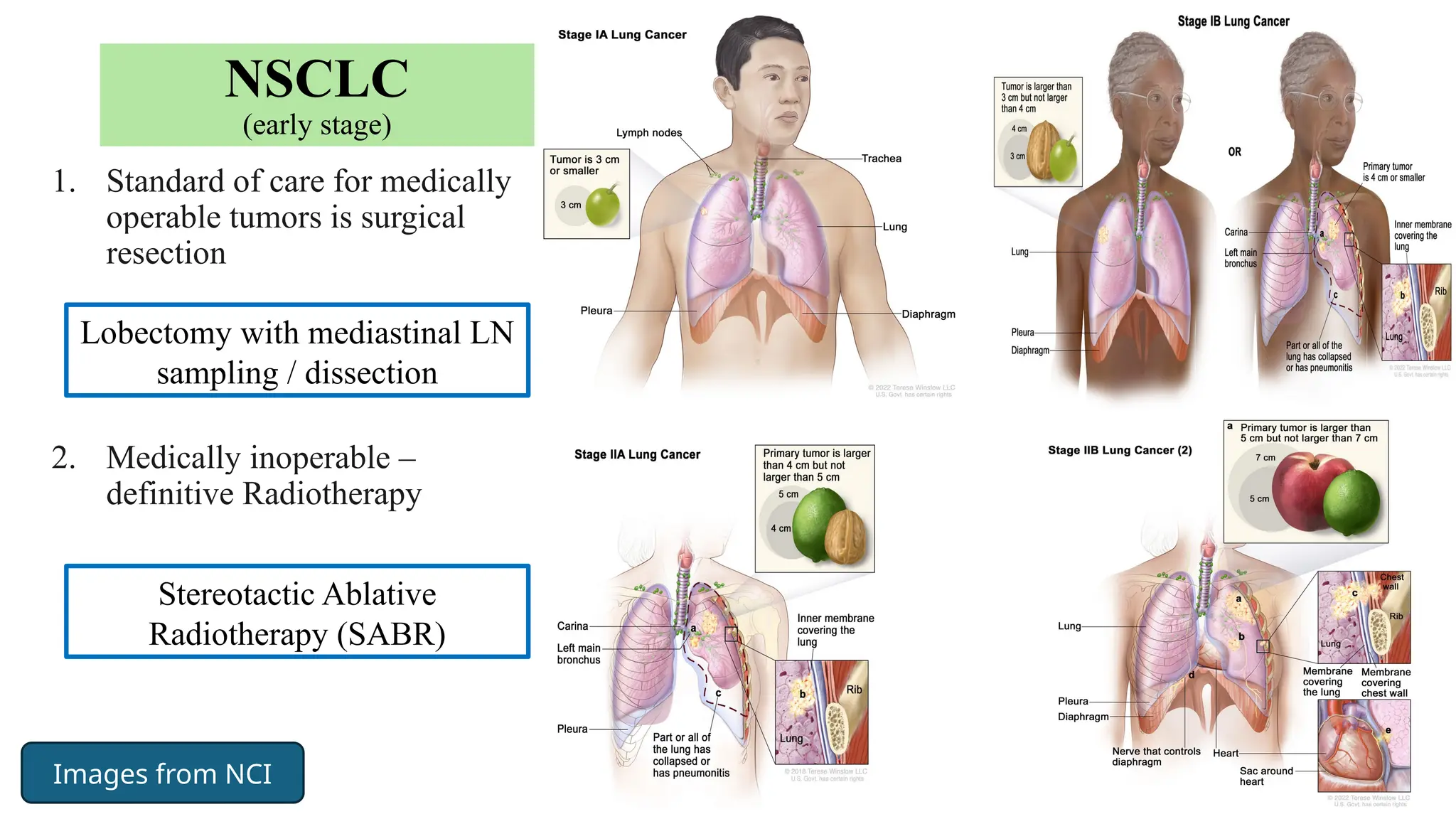
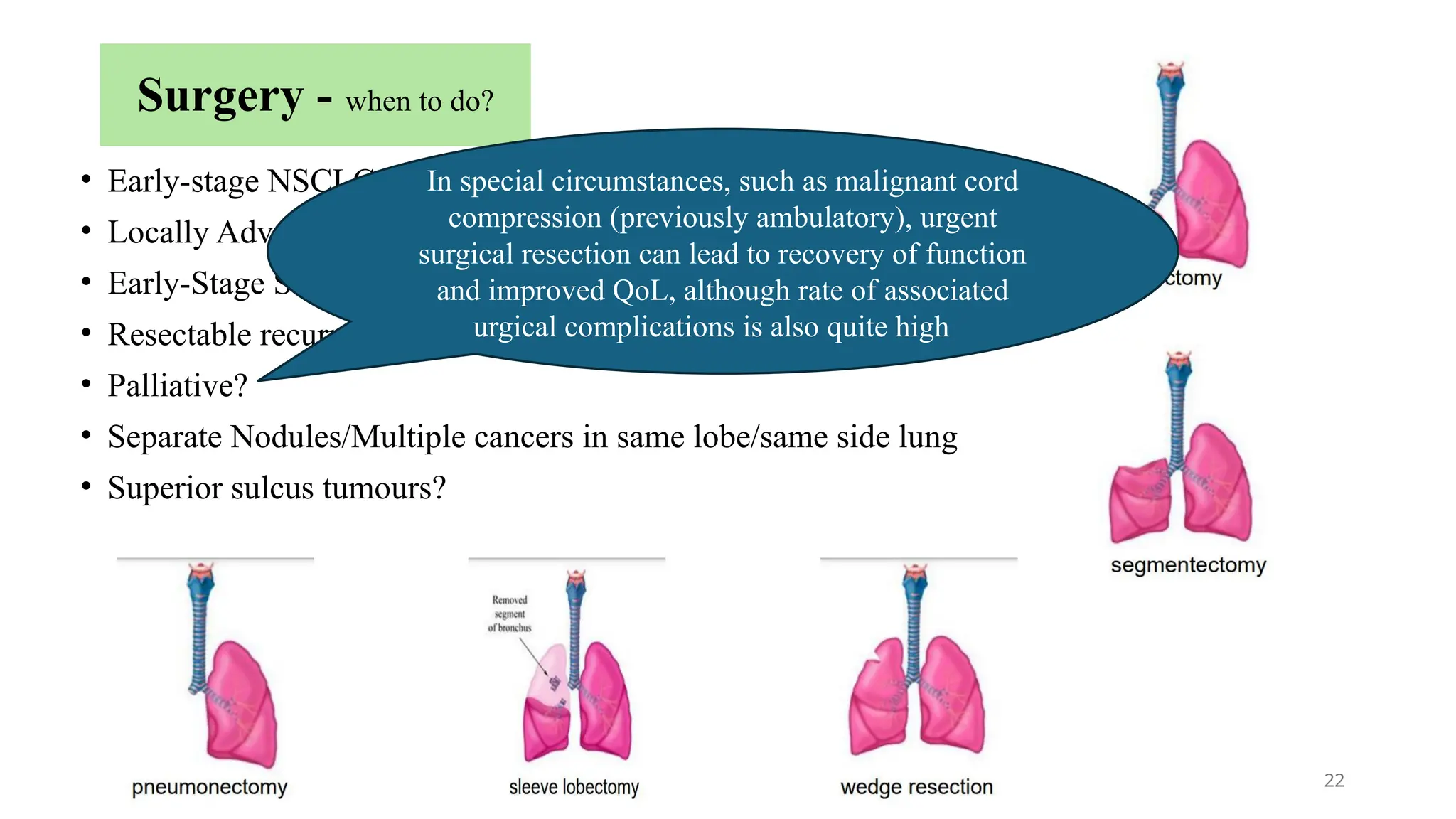
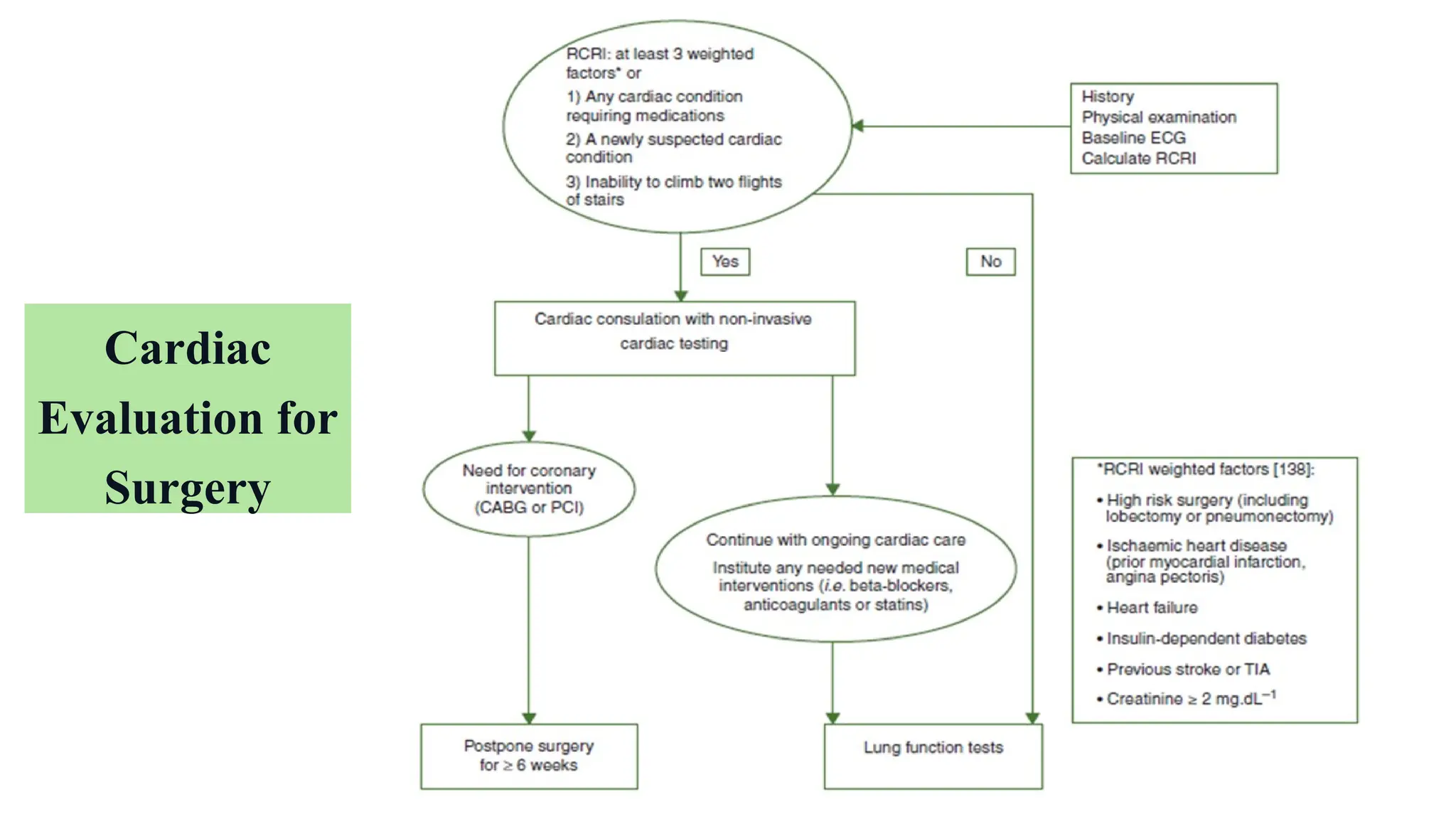
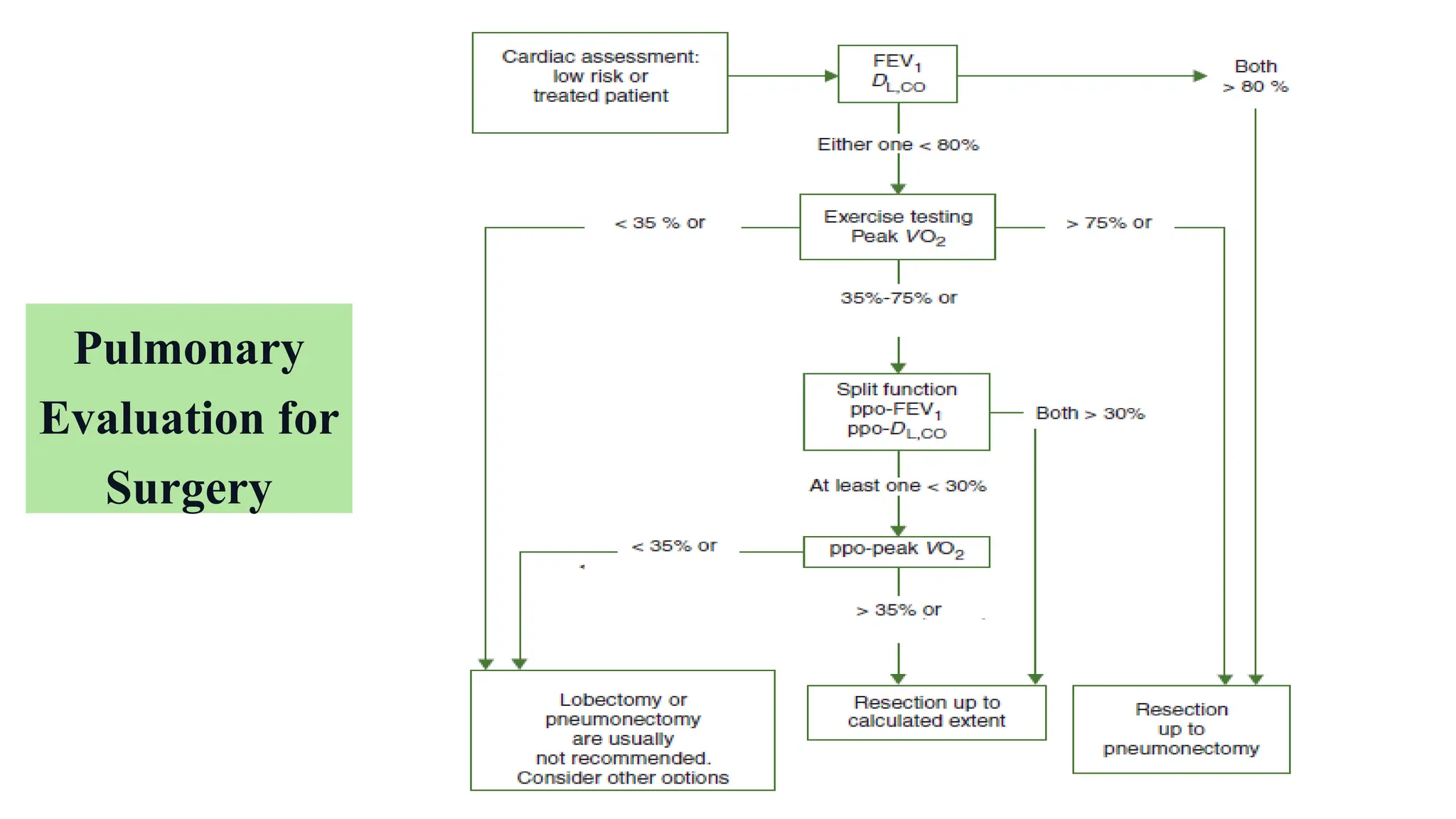
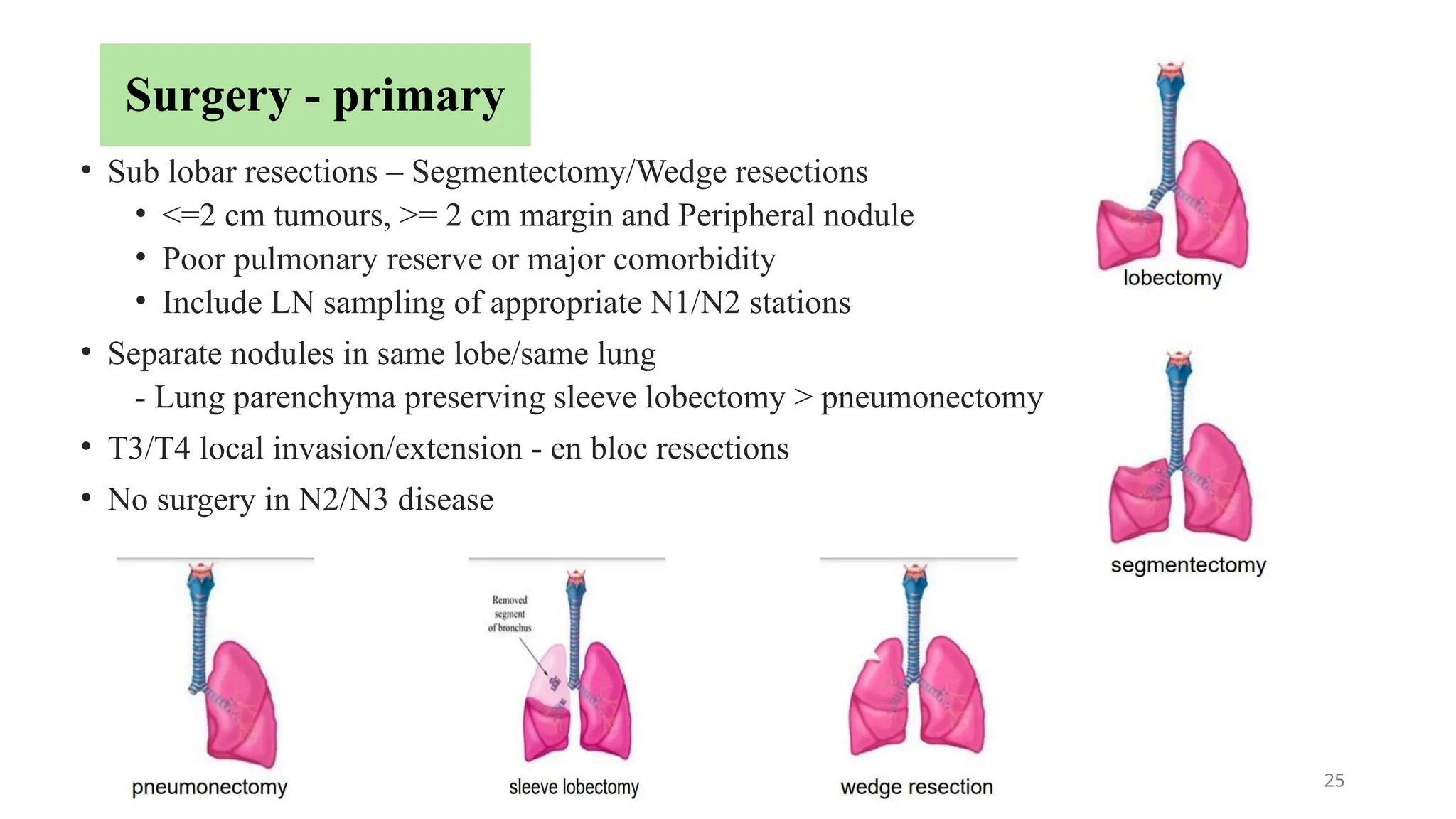
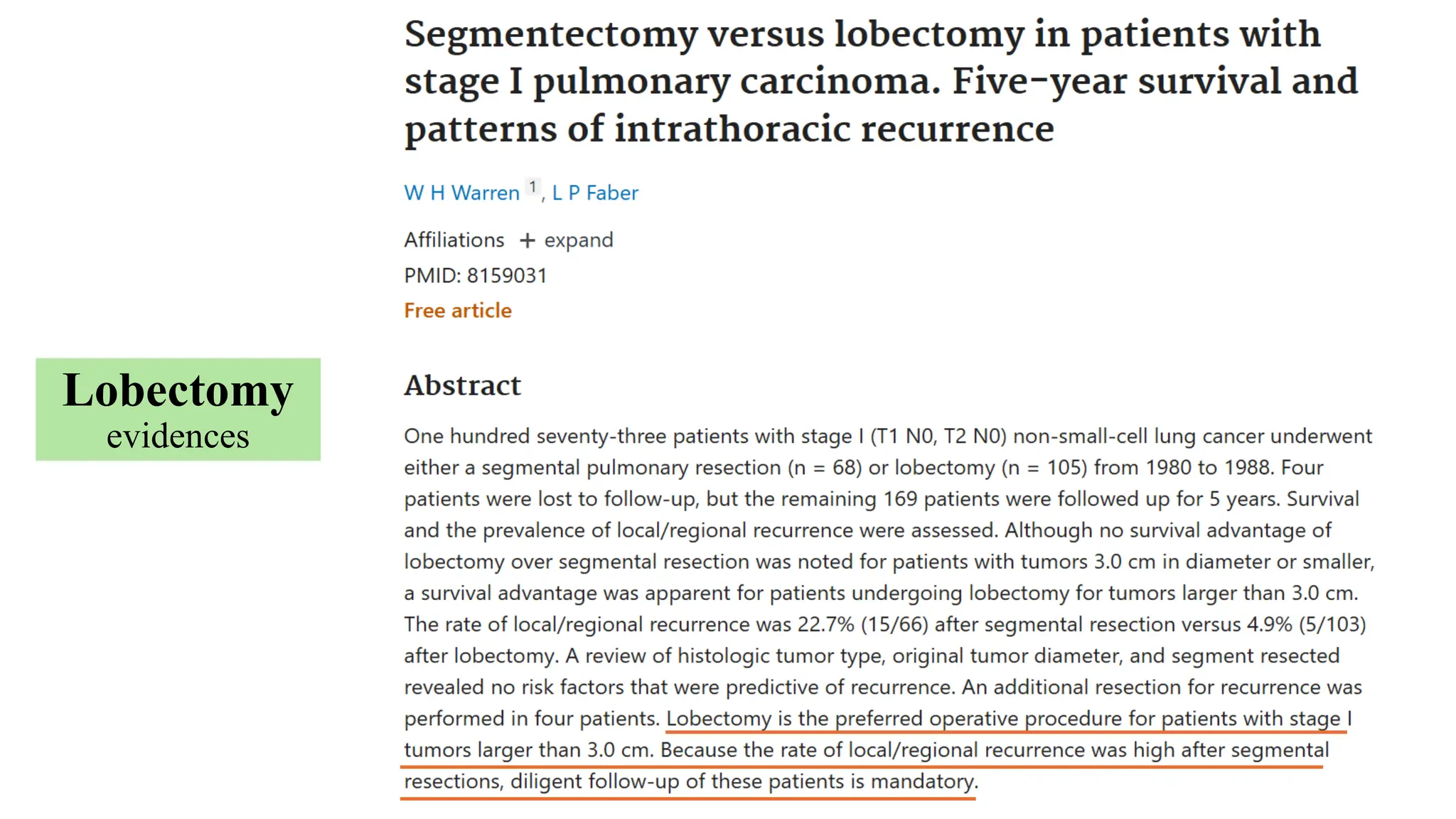
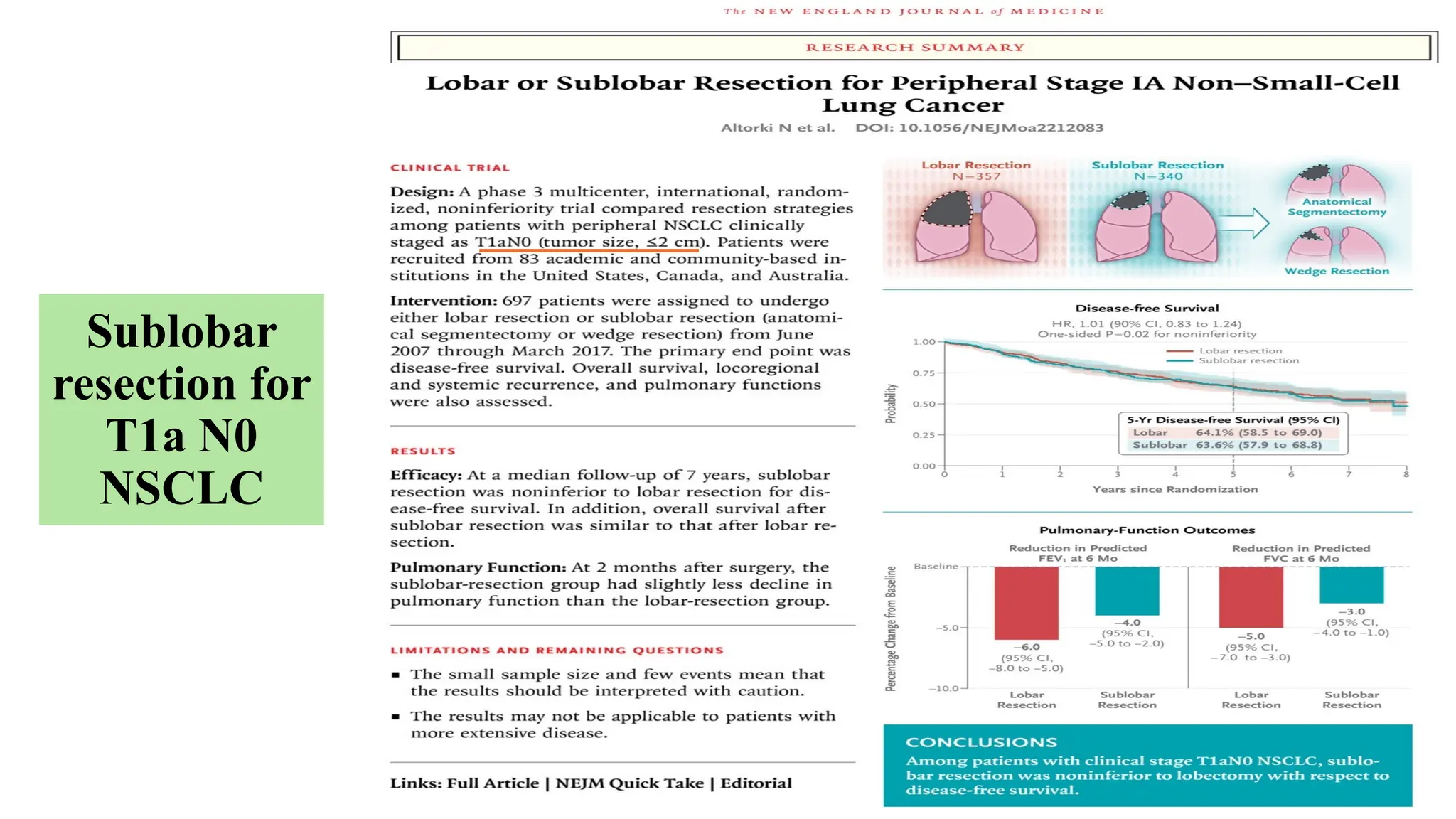
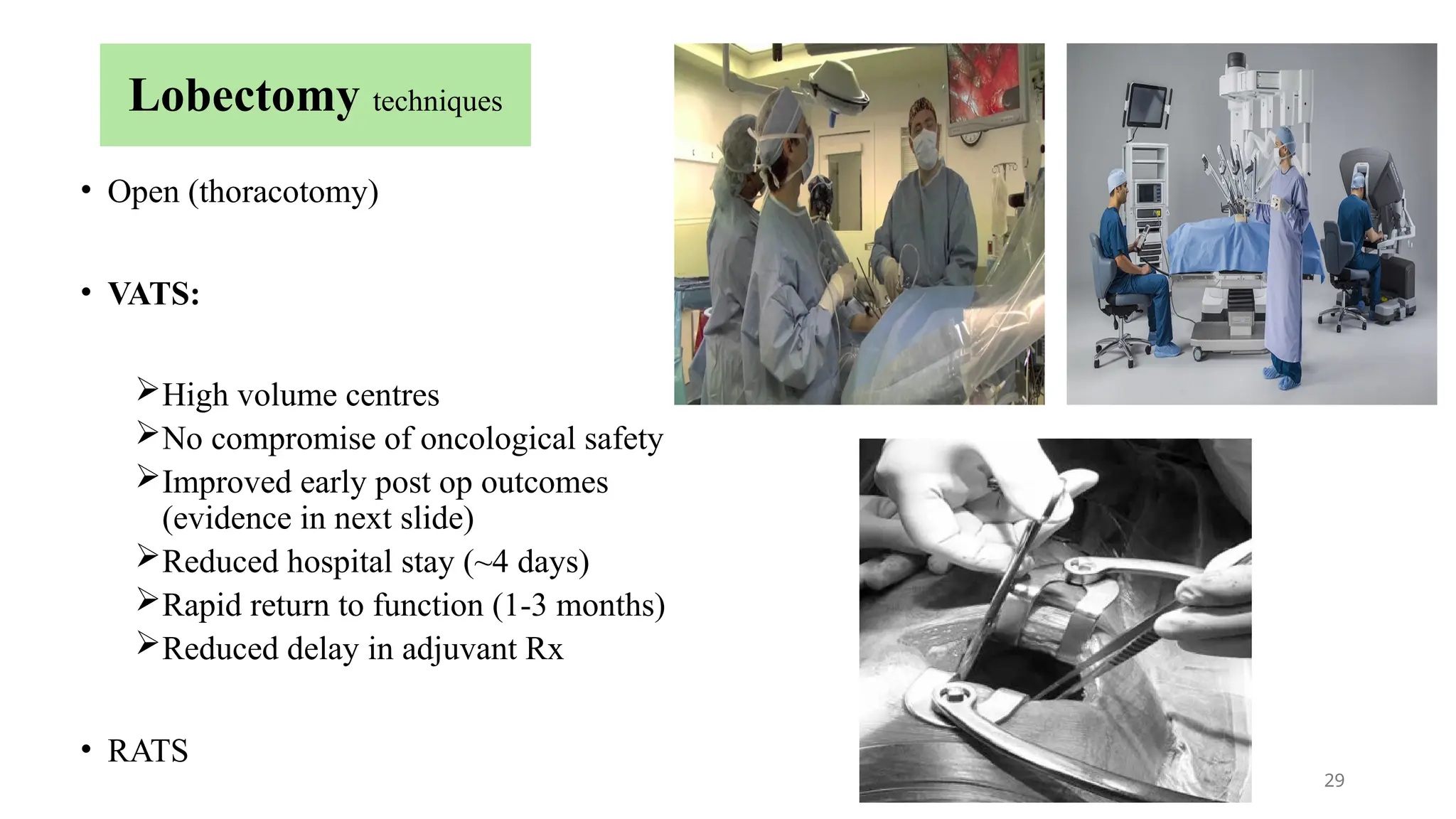
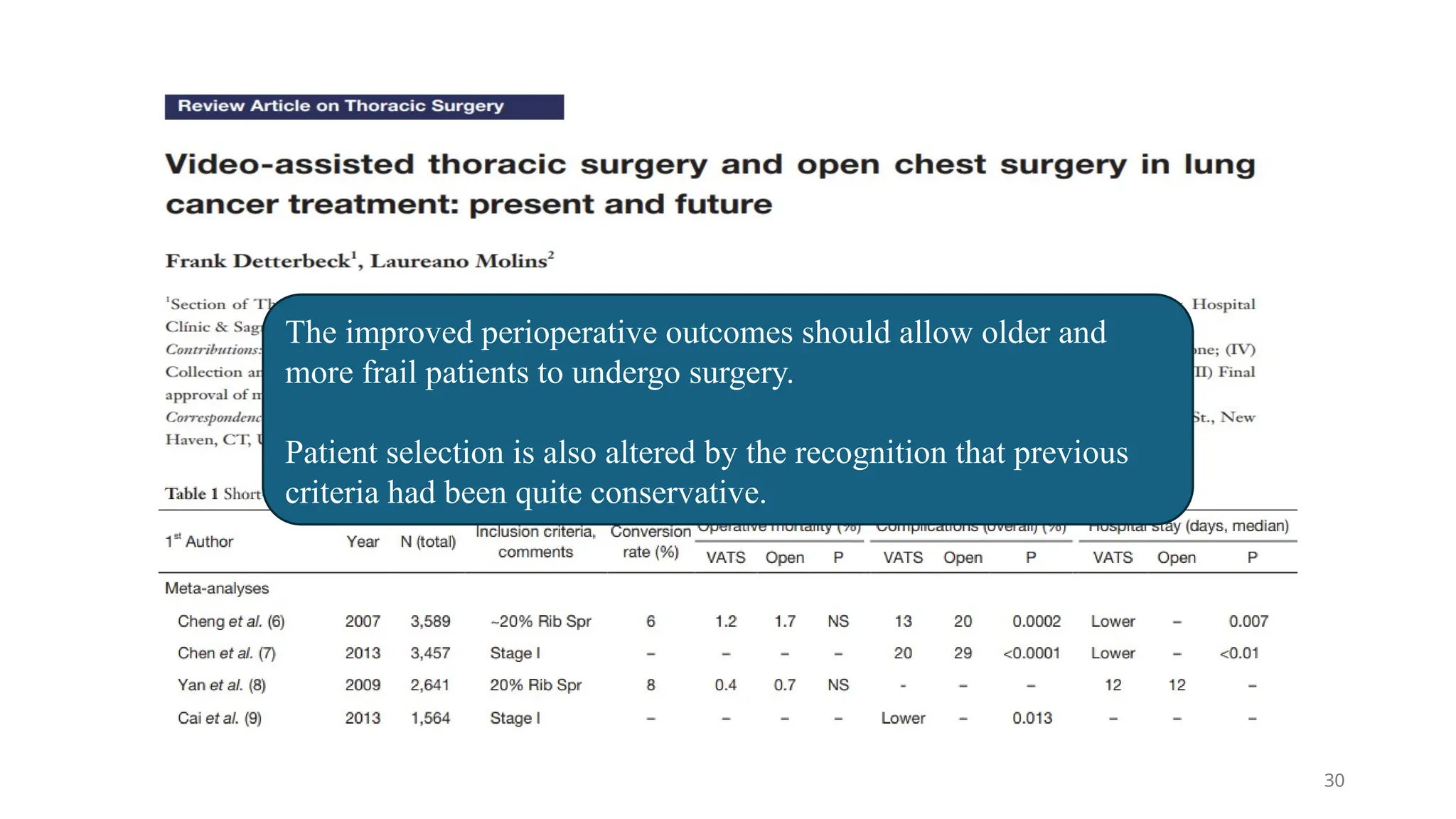
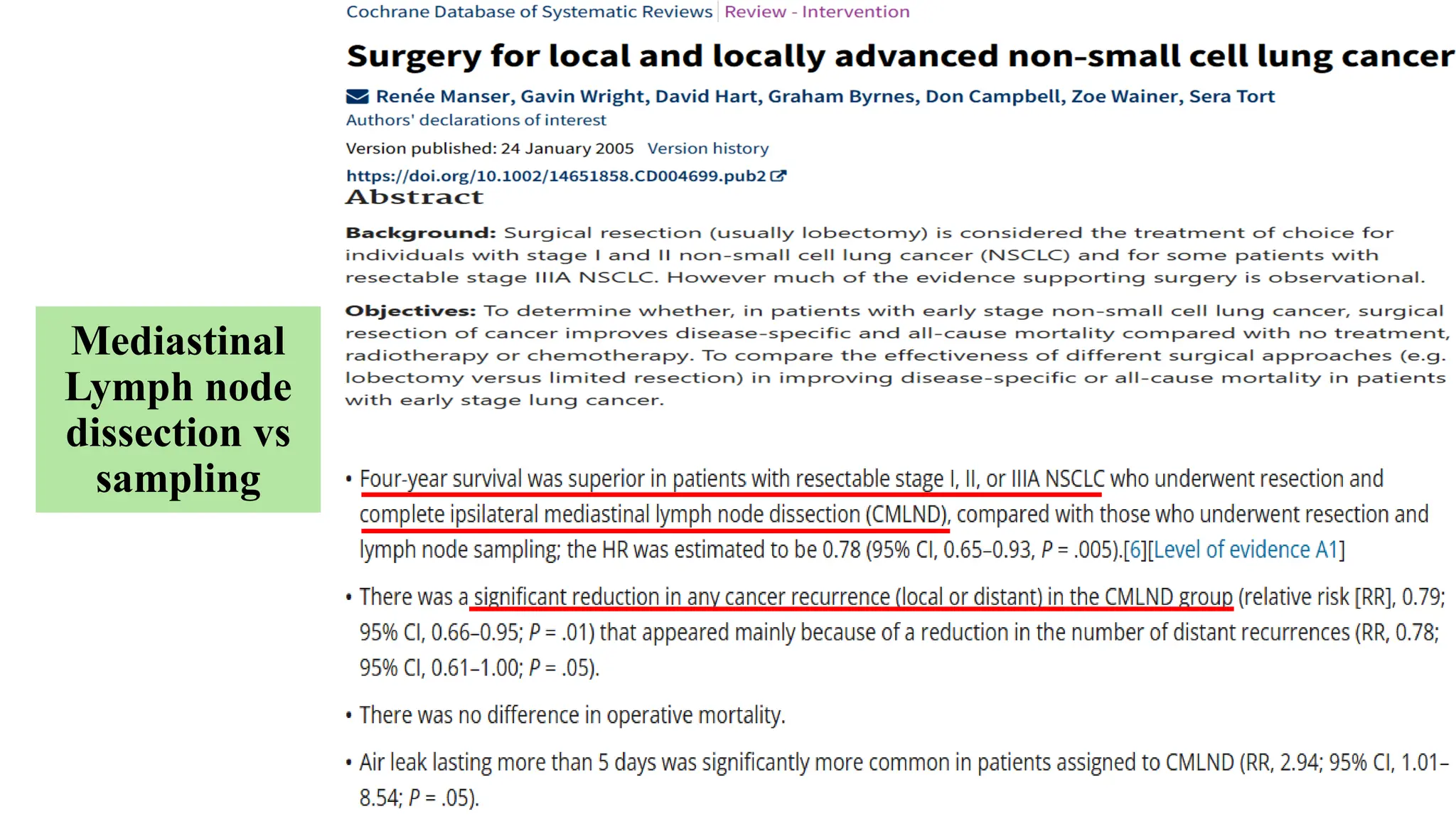
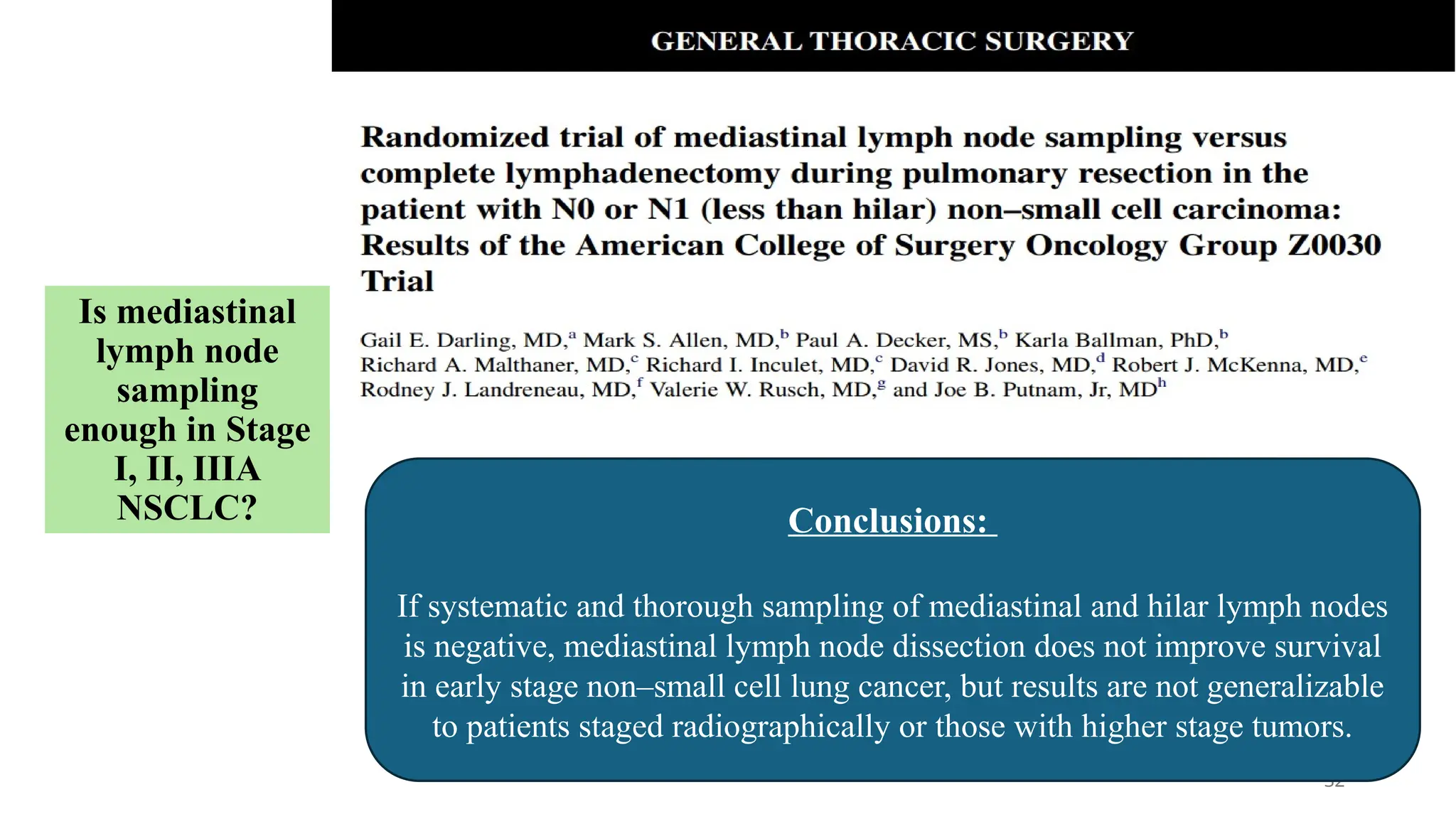
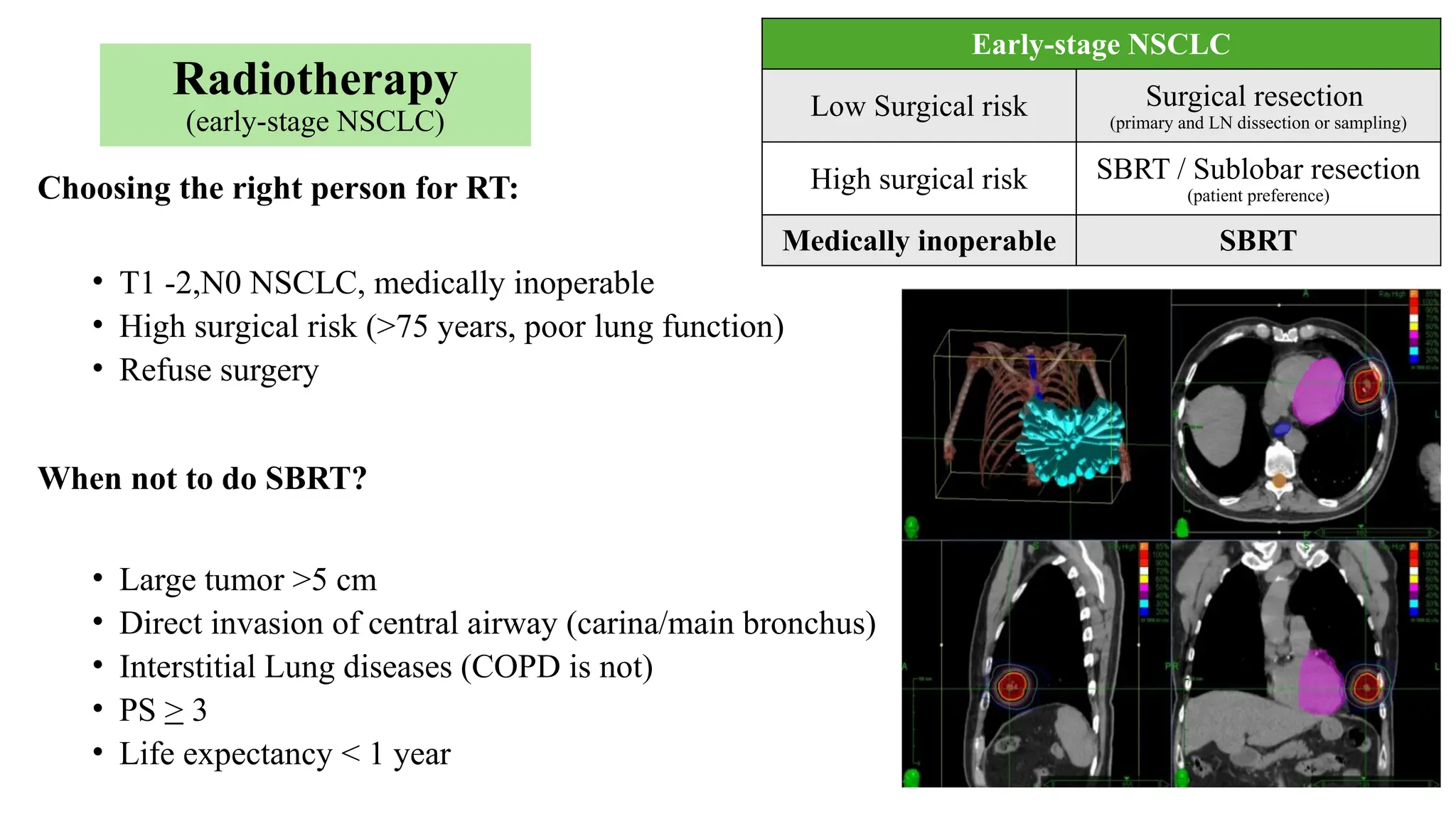
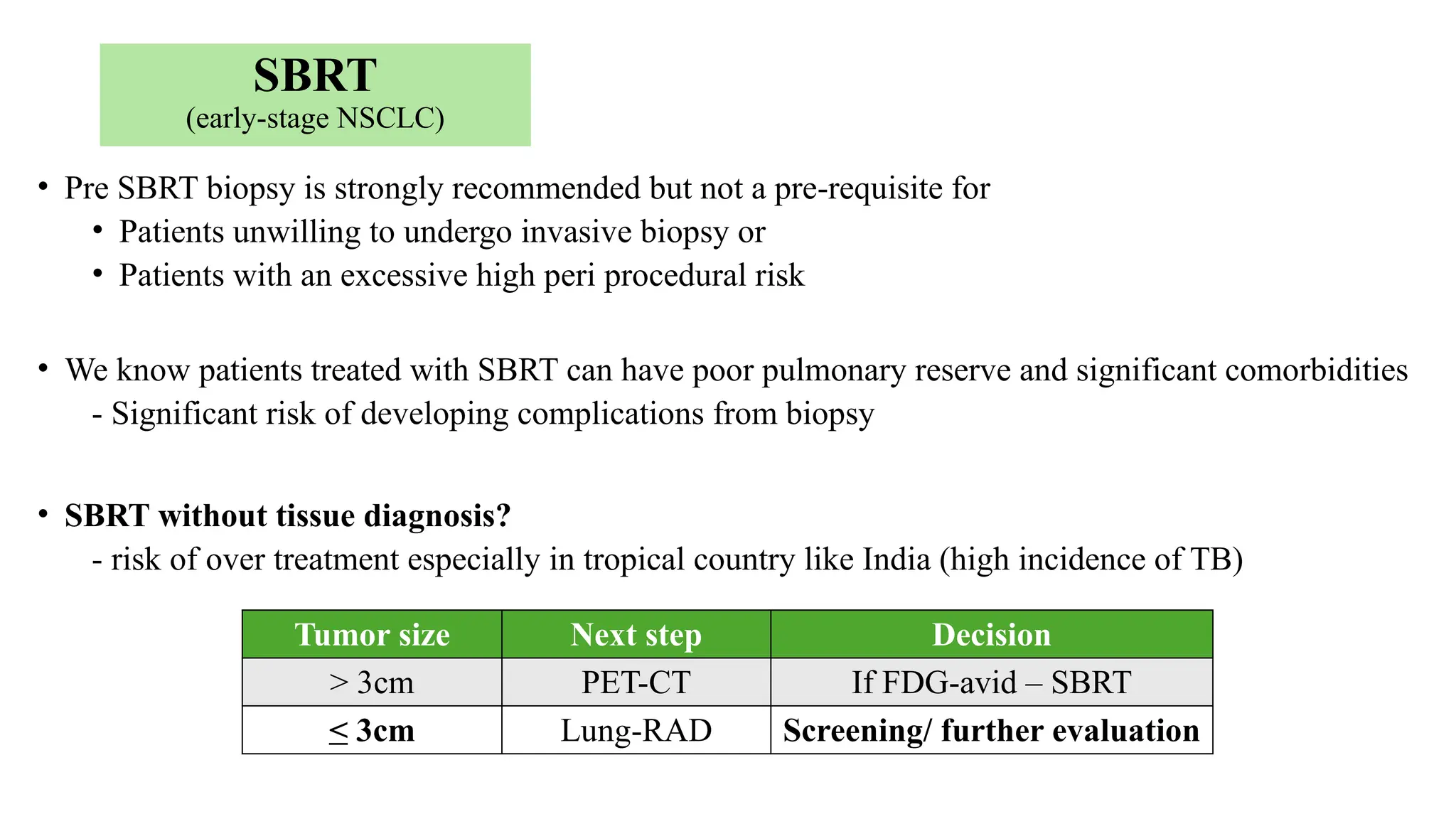
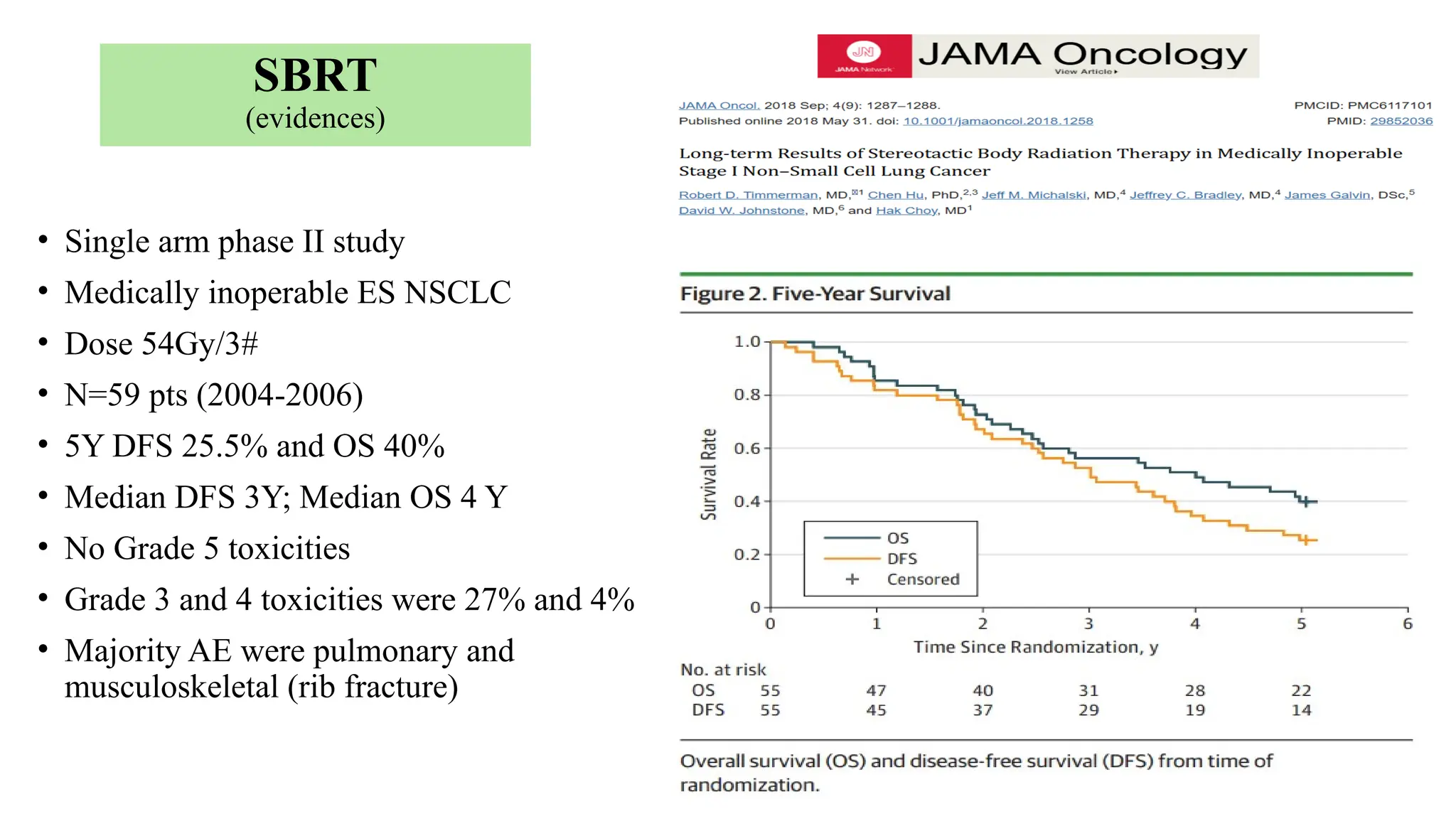
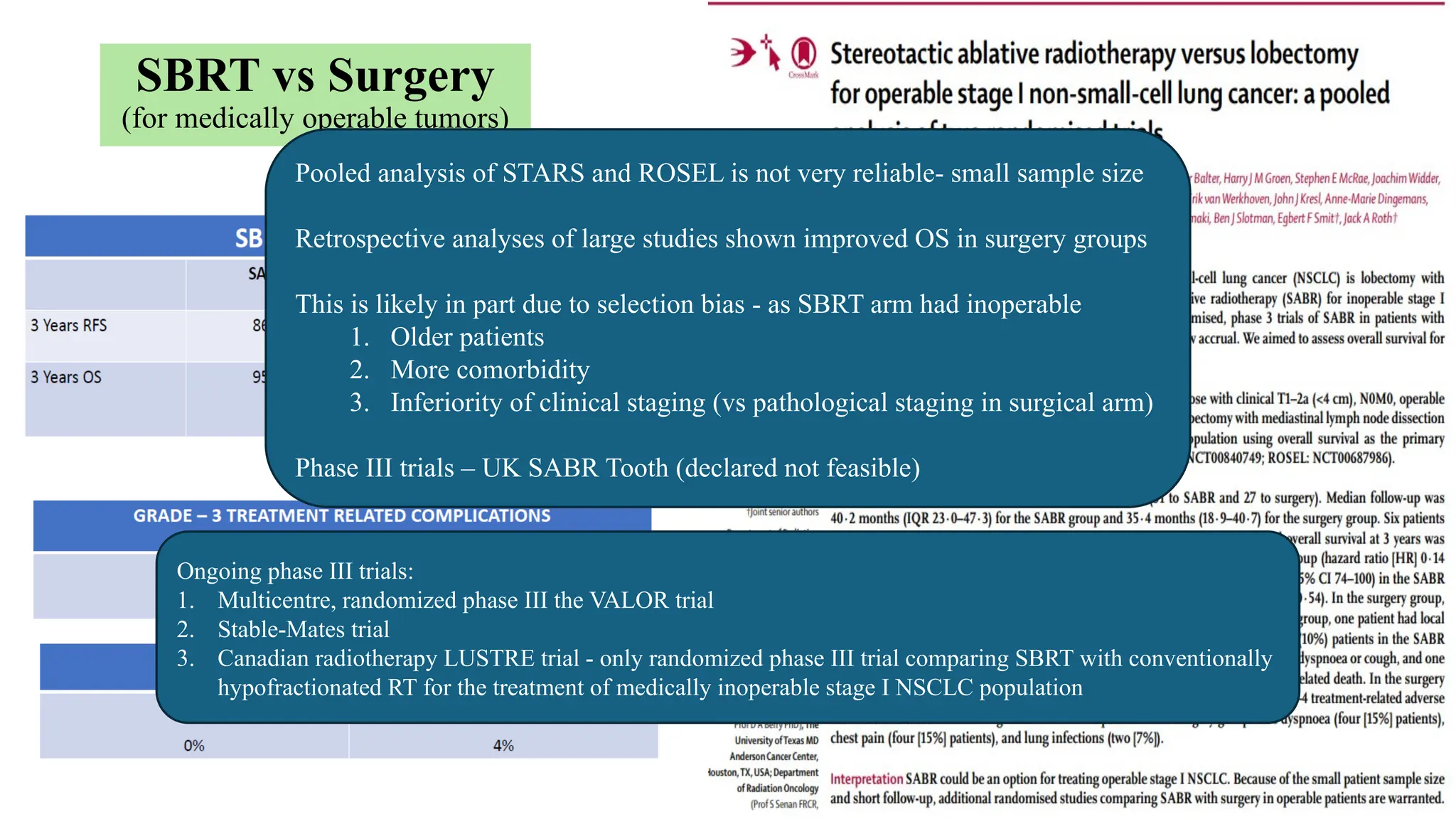
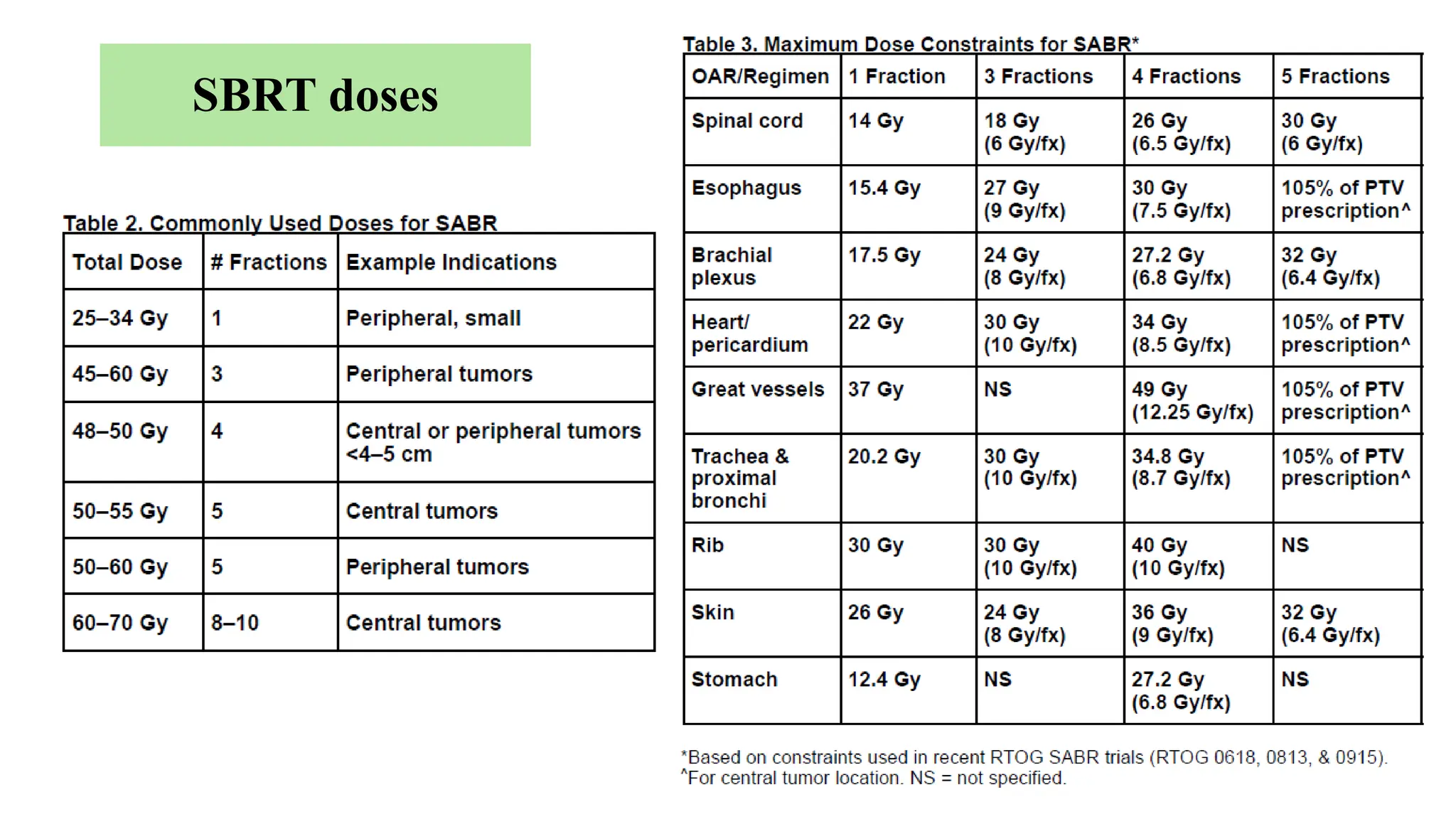
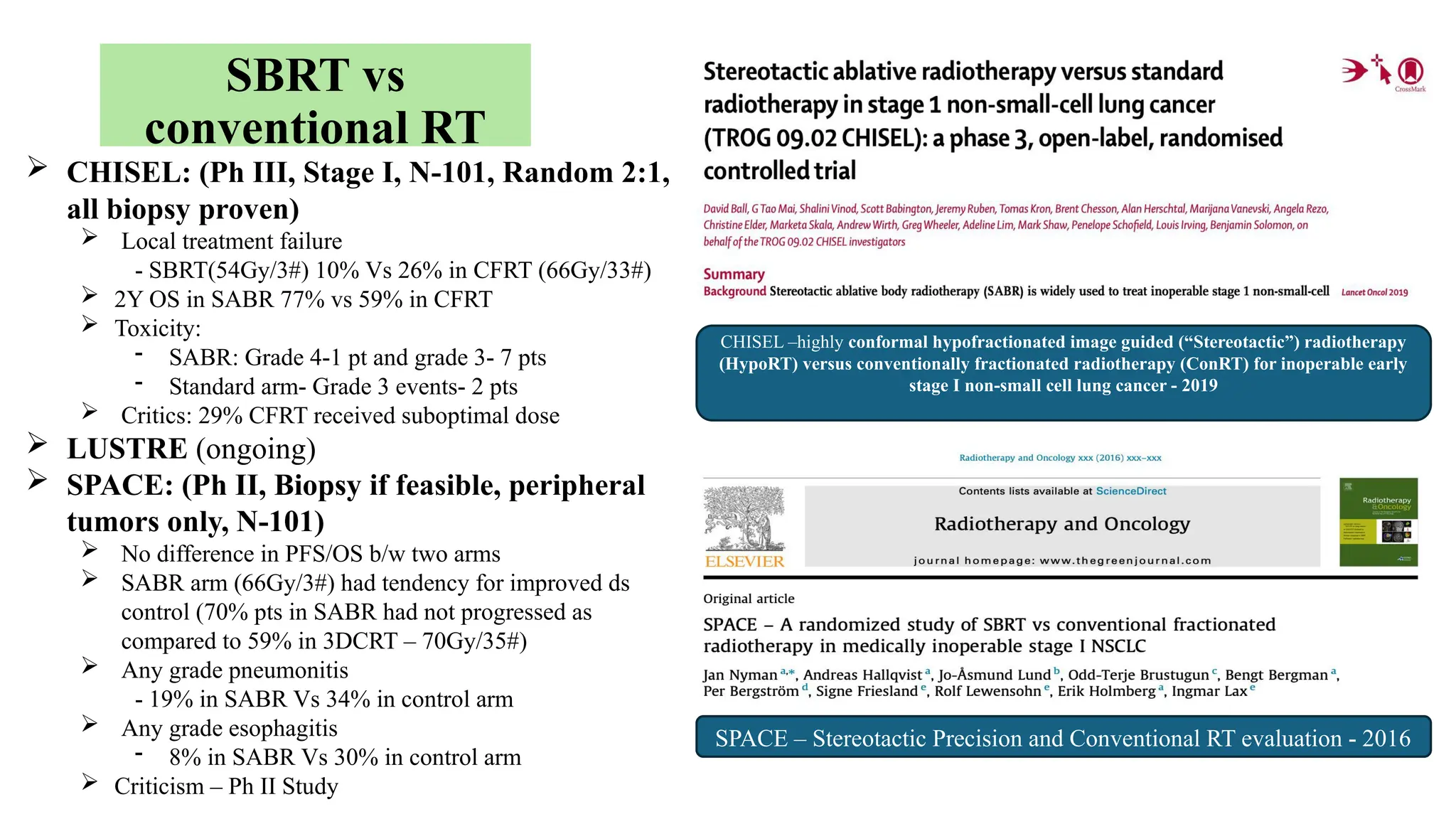
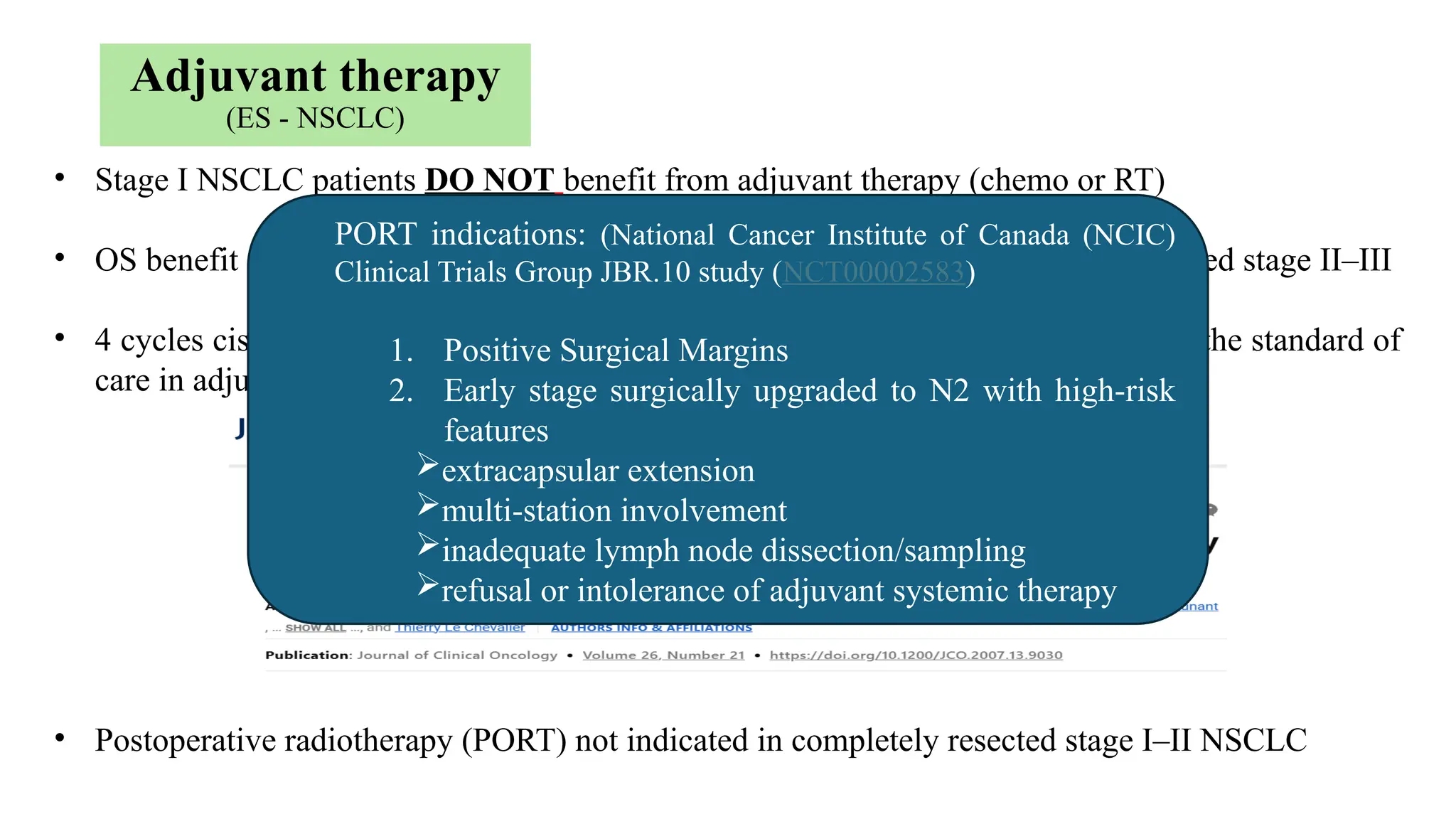
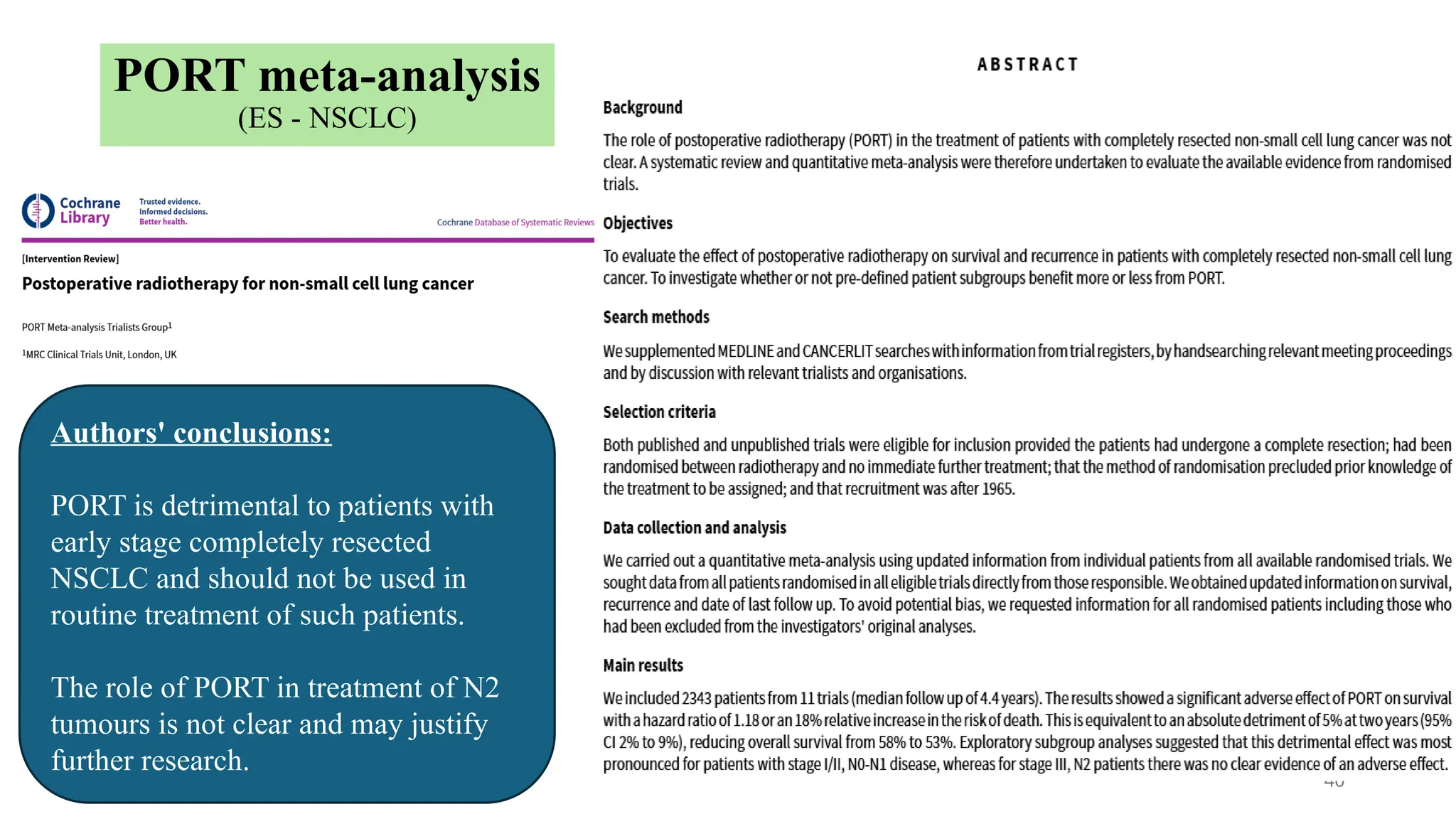
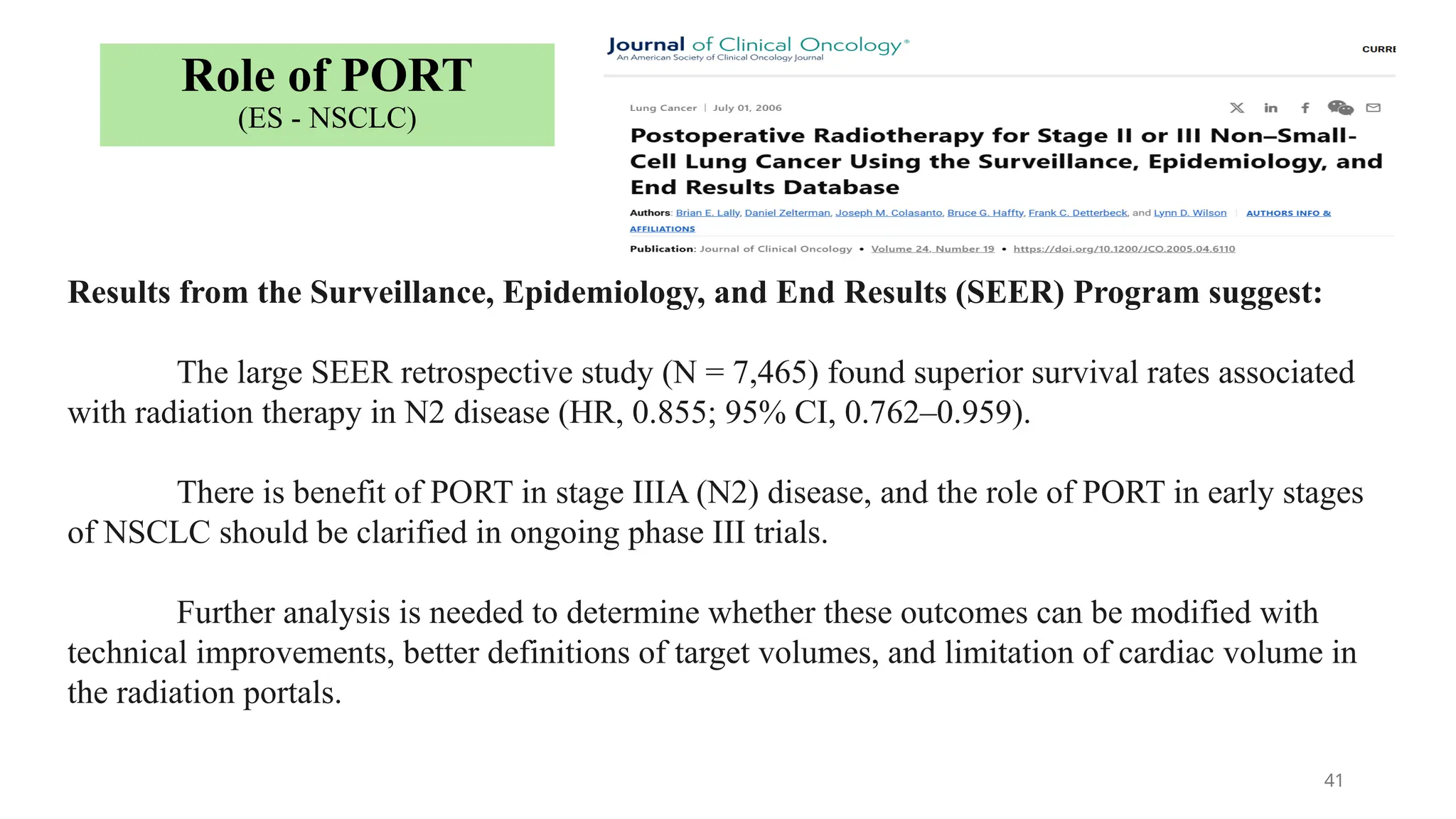
The document presents a comprehensive overview of lung cancer management, focusing on non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC) treatment pathways, including staging, diagnostic workup, and treatment classifications. Key points include the importance of lymph node staging, the role of low-dose CT in screening high-risk individuals, and surgical resection as standard care for early-stage NSCLC. Additionally, it discusses adjuvant therapies, the impact of ongoing clinical trials, and the necessity of personalized treatment based on patient risk factors.


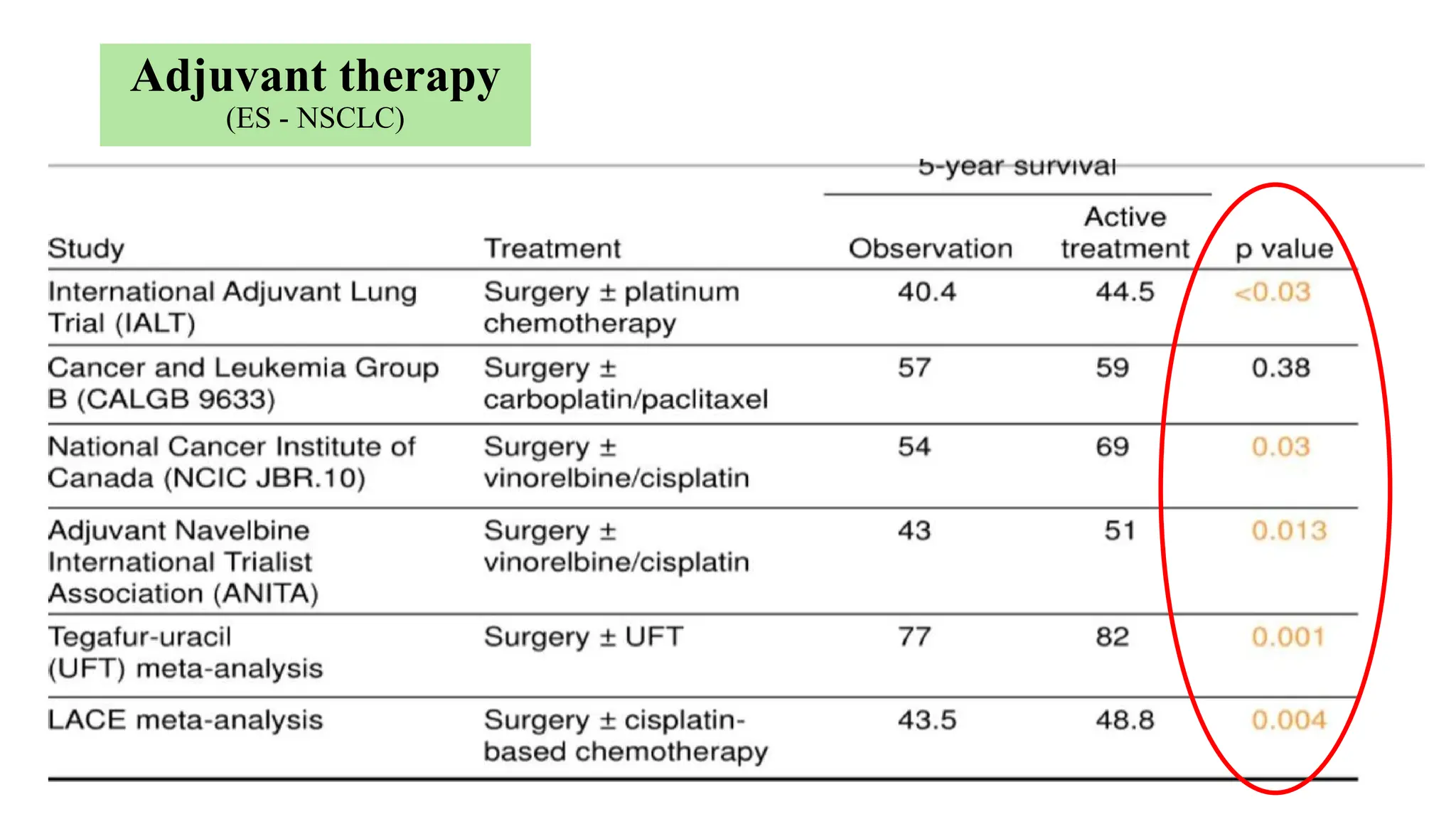
![Adjuvant CTH
(ES - NSCLC)
High risk features:
1. Poorly differentiated tumors (including lung
neuroendocrine tumors [excluding well-differentiated
neuroendocrine tumors])
2. Vascular invasion
3. Wedge resection
4. Visceral pleural involvement
5. Unknown lymph node status (Nx).](https://image.slidesharecdn.com/presentationlung11-250106152305-ee8c6f53/75/Managment-overview-of-lung-cancer-NSCLC-and-SCLC-42-2048.jpg)