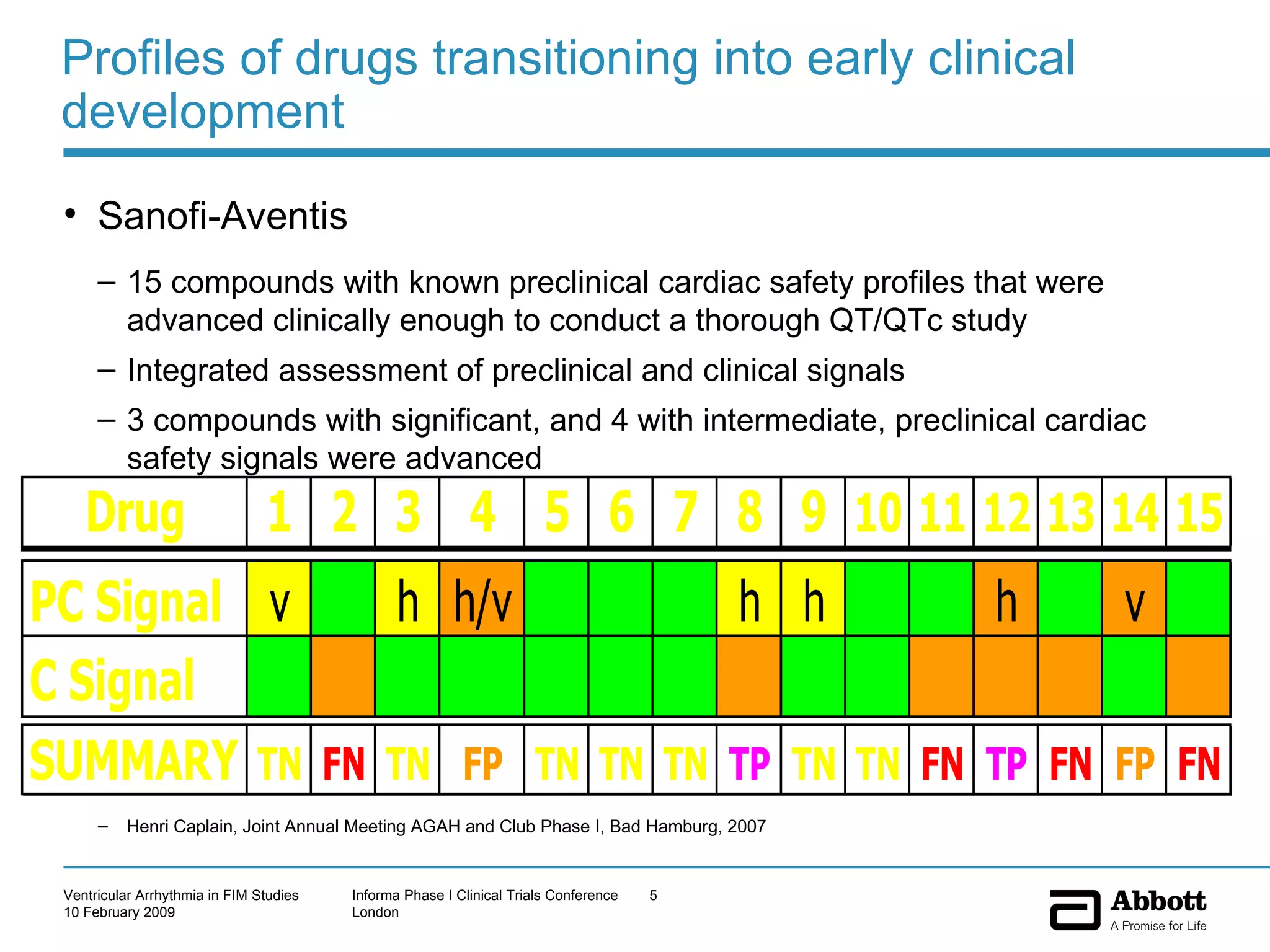
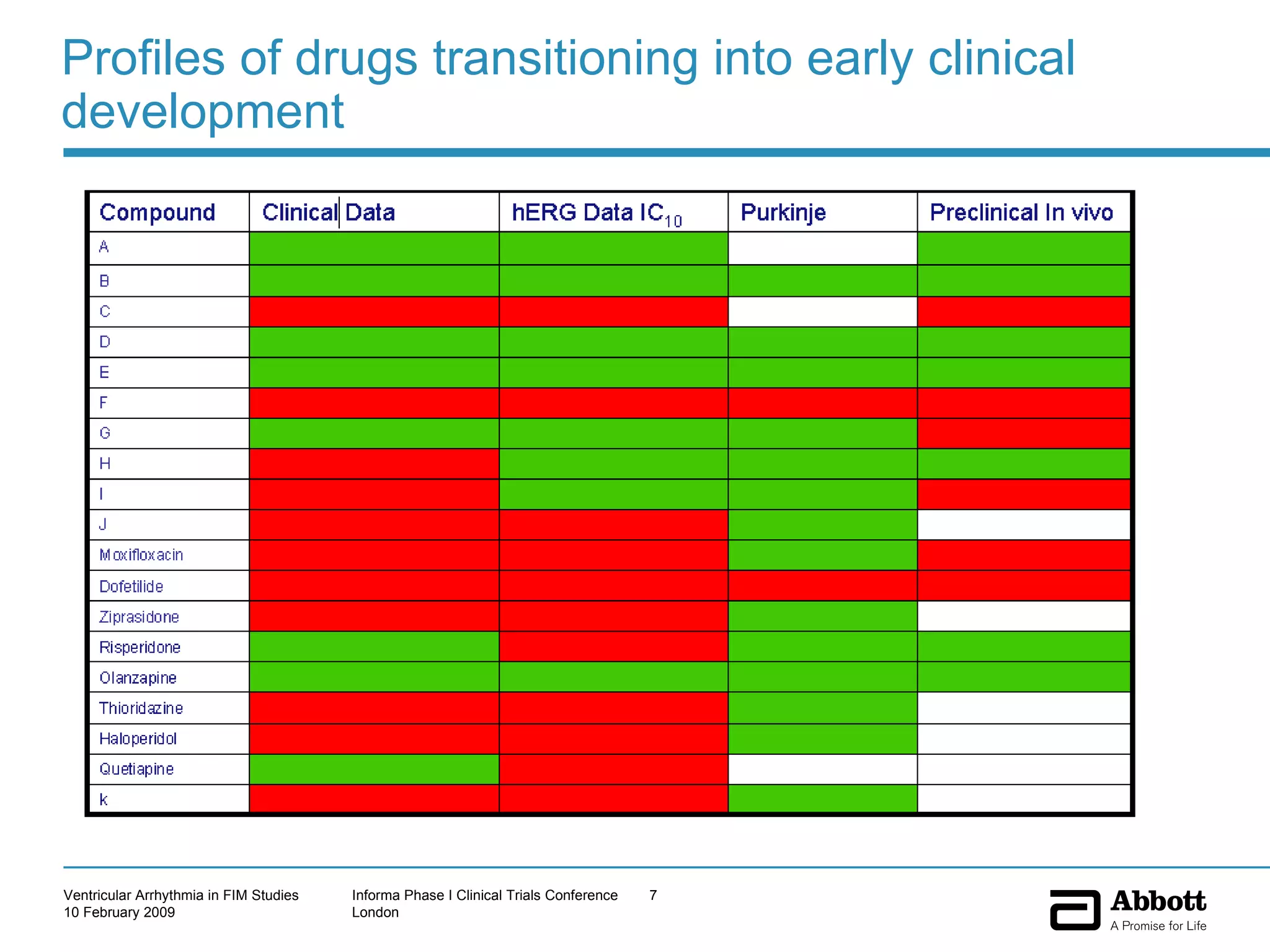
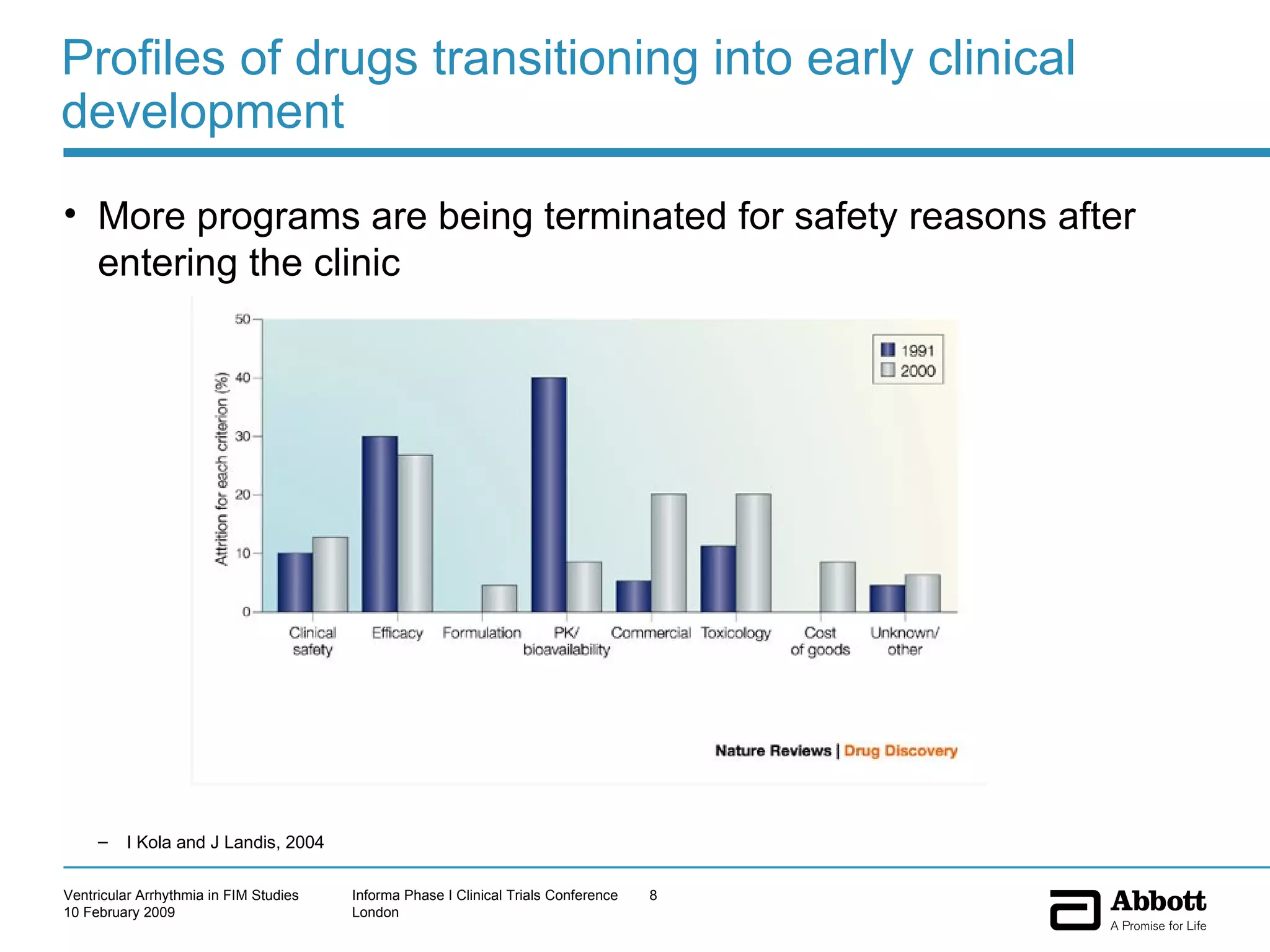
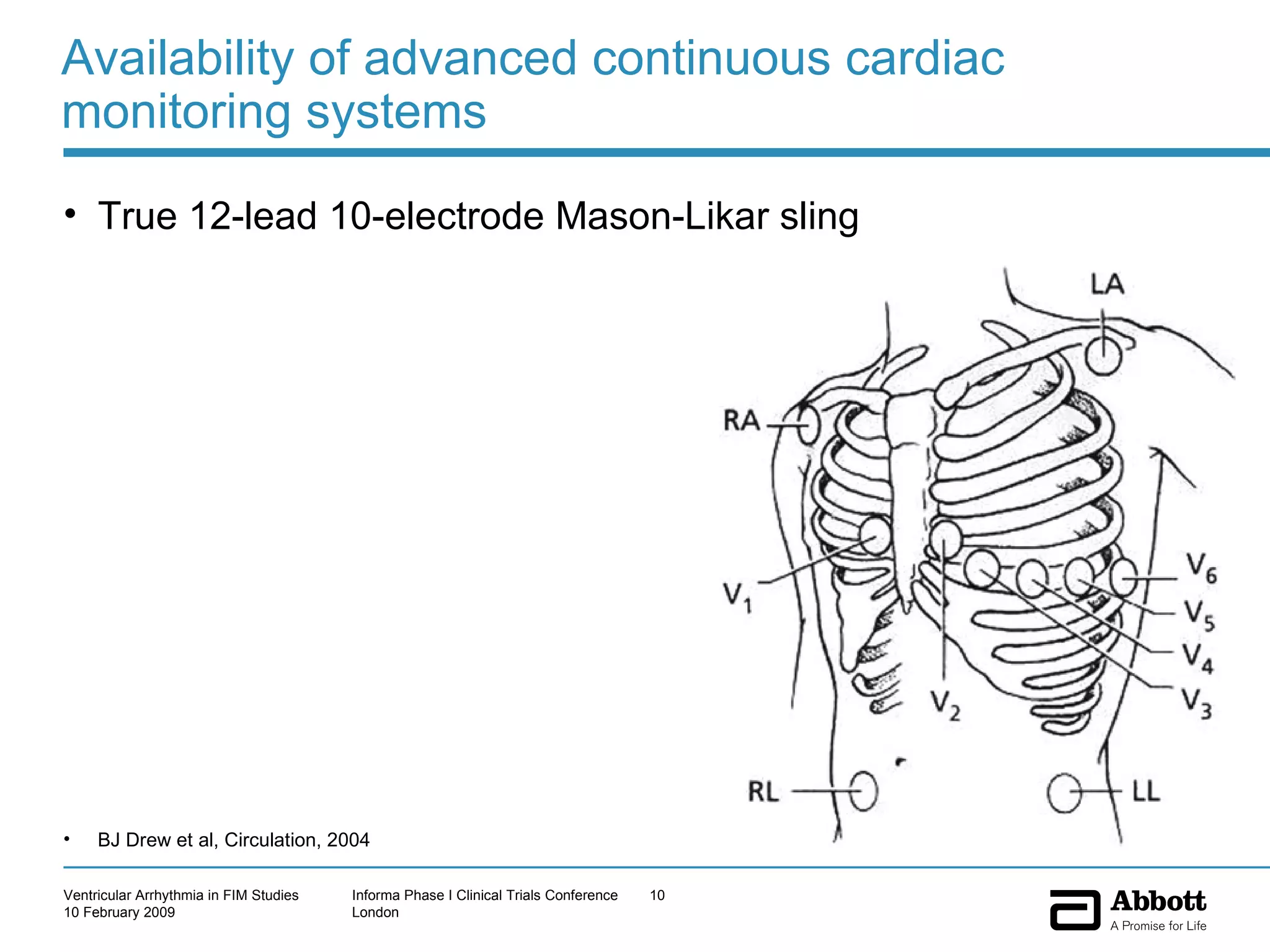
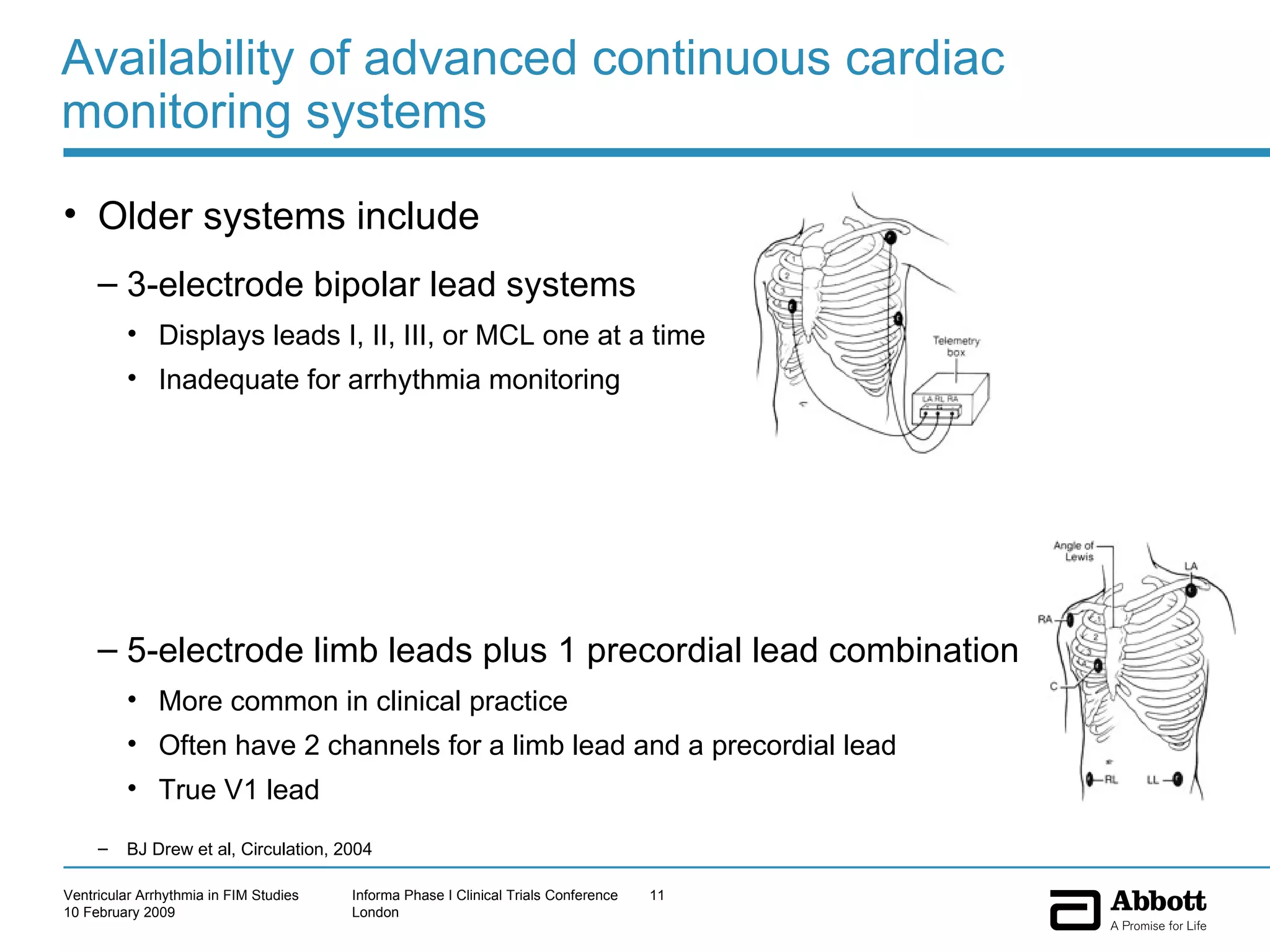
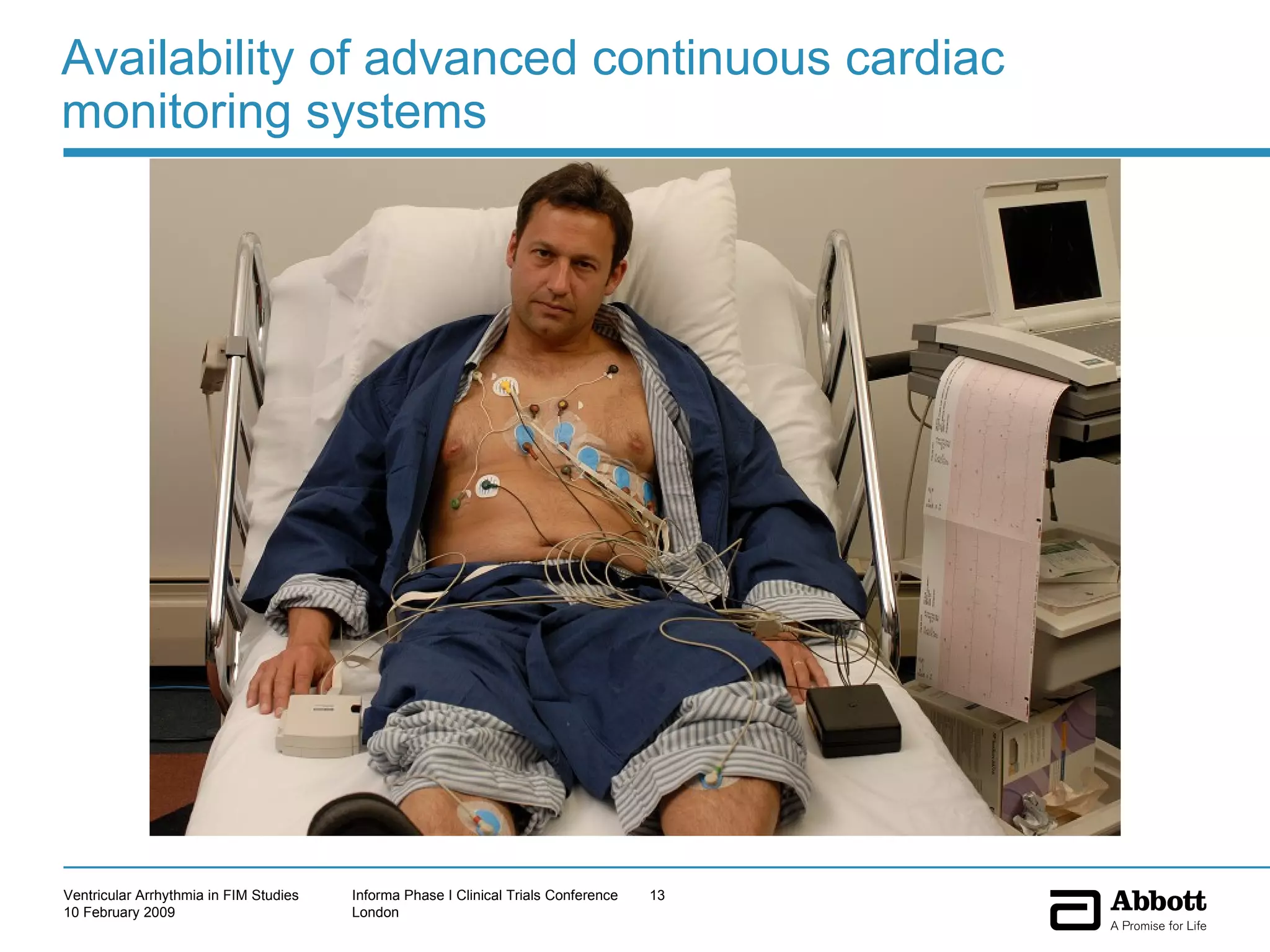
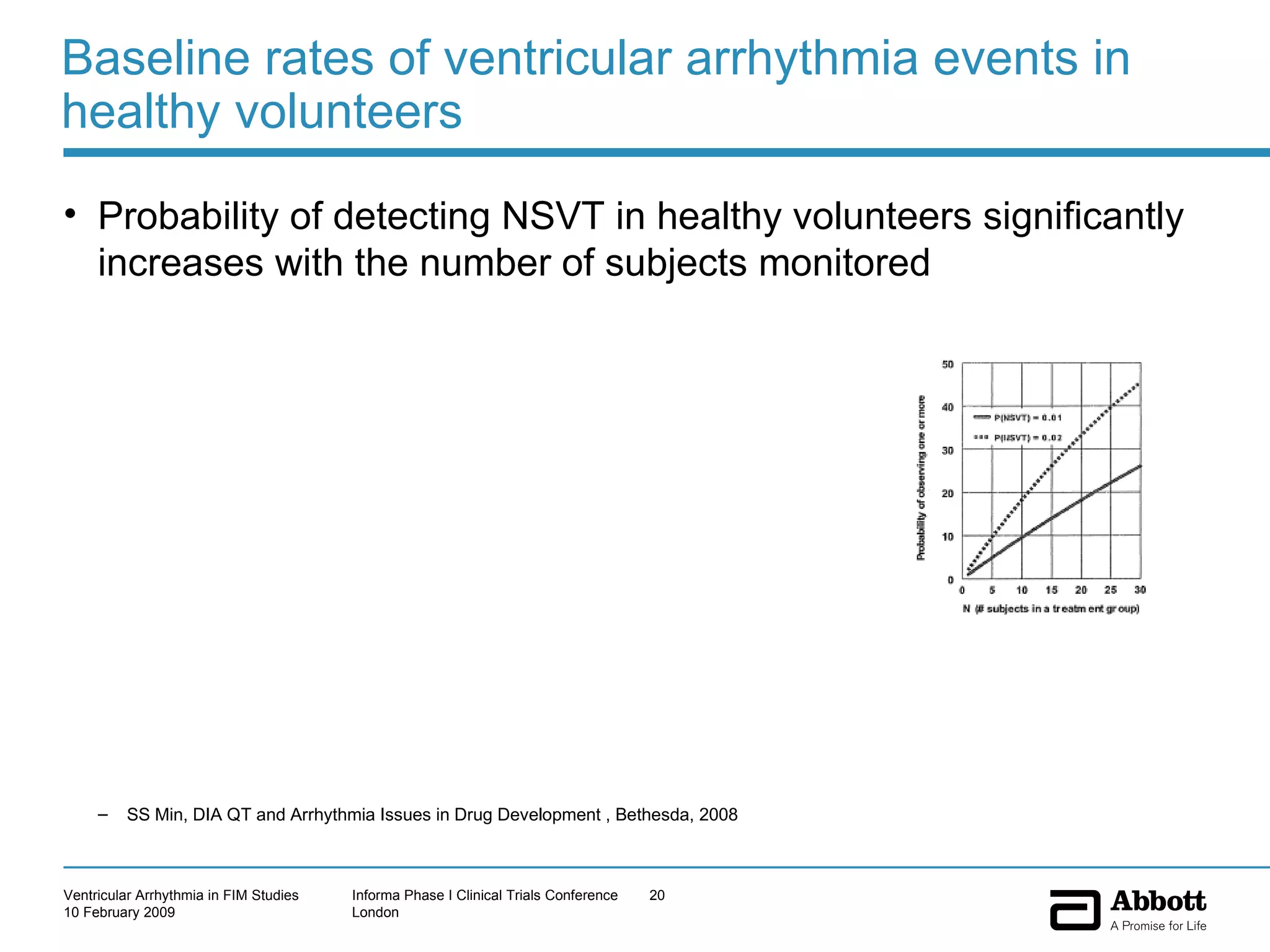
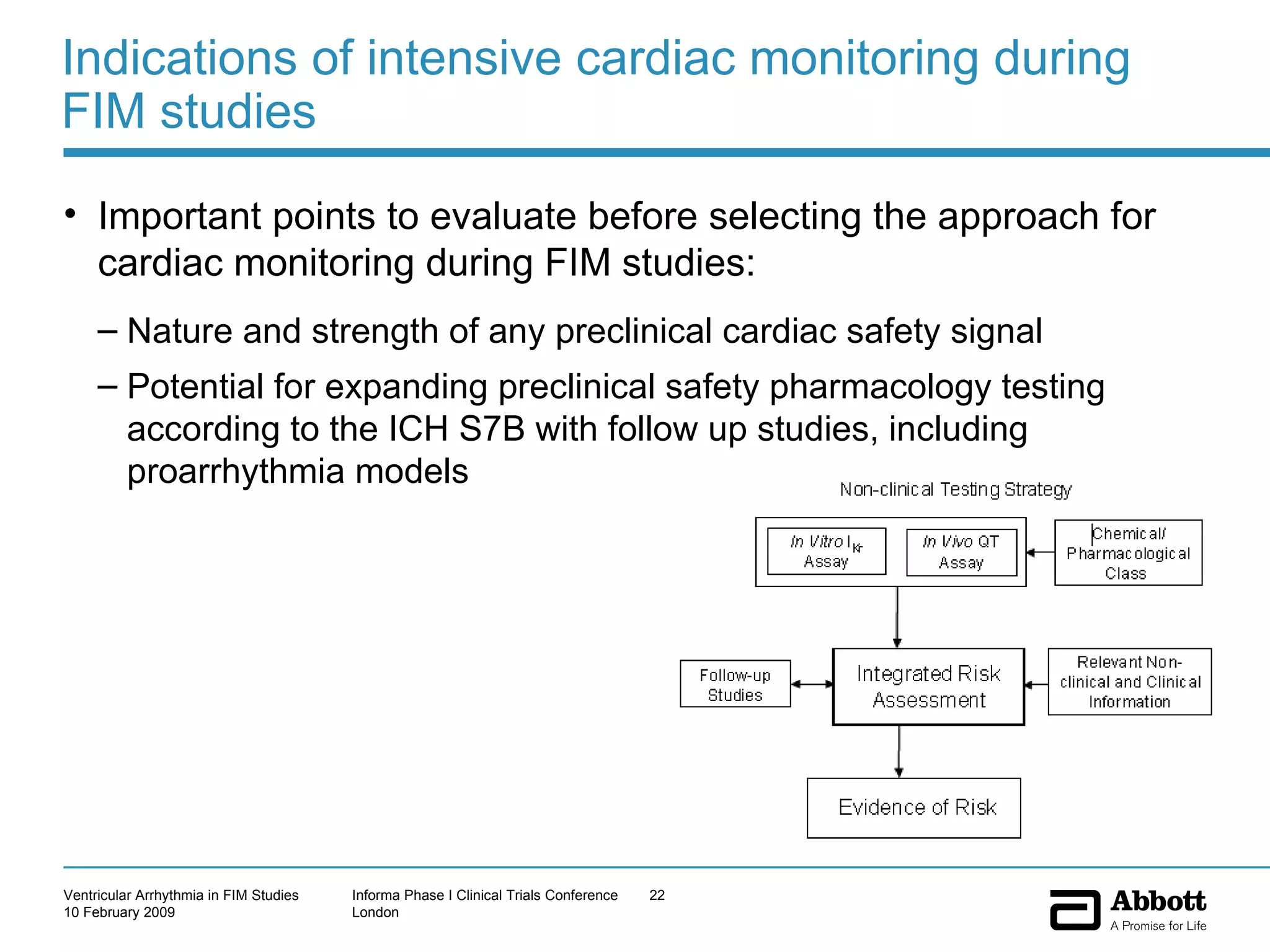
The document discusses the increasing need to manage ventricular arrhythmia encountered during early clinical drug development as riskier compounds are advanced. It outlines factors contributing to this issue and strategies for intensive cardiac monitoring in first-in-man studies to ensure safety while accurately assessing potential drug-related arrhythmia events given baseline rates in healthy volunteers. Future directions are highlighted, including expert guidance documents and initiatives to better understand cardiac safety in early development.