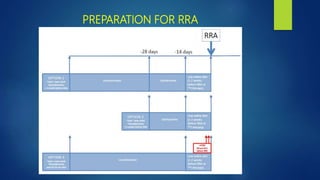
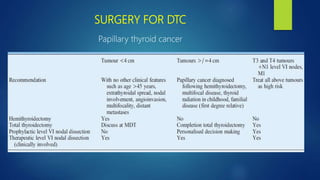
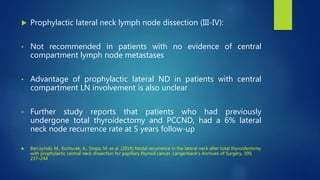
This document discusses the management of thyroid malignancies. It covers the goals of treatment, terminology, diagnostic and therapeutic surgery procedures, radioactive remnant ablation and therapy, follow up care, and management of specific thyroid cancers such as papillary microcarcinoma and medullary thyroid carcinoma. Key points include that the goals of treatment are to remove the primary tumor and involved lymph nodes while minimizing morbidity, staging the disease accurately, and enabling long term surveillance. It also provides guidance on appropriate surgical procedures and radioactive iodine activities based on risk factors and disease stage.








![ Surgery for follicular thyroid carcinoma [excluding oncocytic
(Hurthle cell) follicular carcinoma]:](https://image.slidesharecdn.com/seminar-managementofthyroidmalignancies-181006101543/85/Management-of-thyroid-malignancies-11-320.jpg)