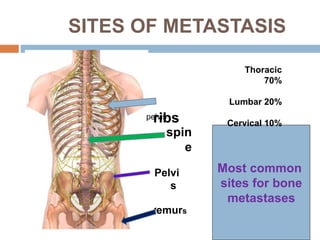
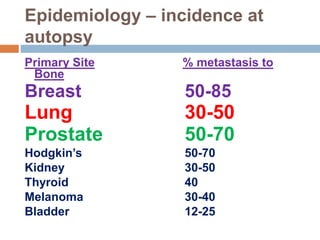
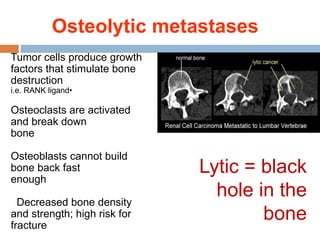
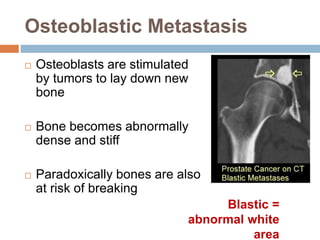
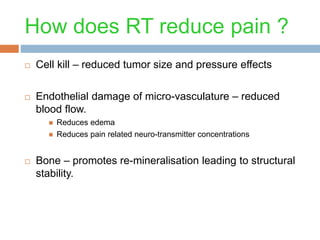
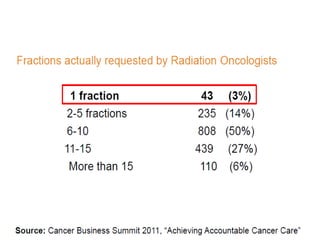
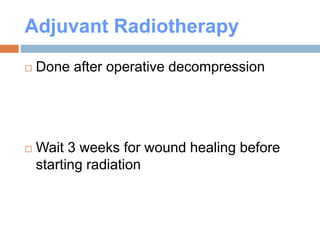
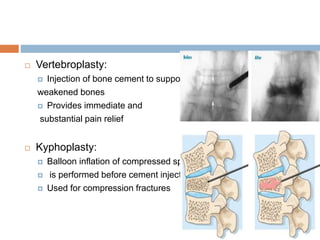
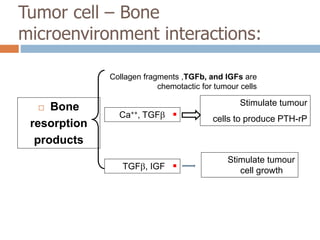
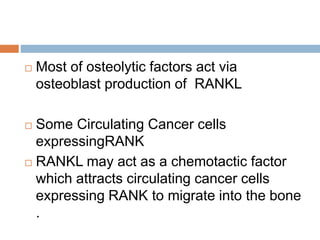
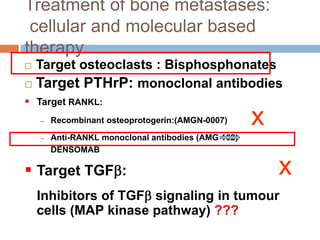
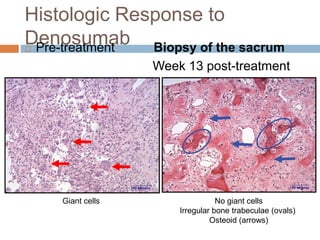
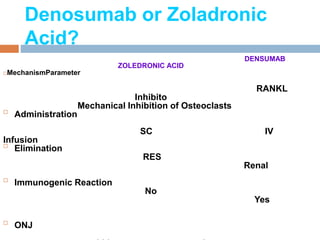
This document discusses the management of bone metastases. It begins by explaining how tumor cells interact with bone cells, disrupting normal bone metabolism and increasing osteoclast activity. This leads to skeletal complications over several years for cancers like myeloma, breast, and prostate. Common sites of bone metastases are then outlined. Treatment options discussed include systemic therapies like bisphosphonates and denosumab which target osteoclasts and RANKL, as well as local therapies like surgery, radiation, vertebroplasty, and kyphoplasty. Denosumab is positioned as an alternative to zoledronic acid, with potential advantages of subcutaneous dosing and reduced risks of osteonecrosis of the jaw and renal toxicity. Guidelines recommend













![Denosumab vs Zoledronic Acid
Pivotal Phase III SRE Prevention
Trials
In total, > 5700 patients with bone
metastases
R
A
N
D
O
M
I
Z
A
T
I
O
N
Denosumab 120 mg SC q4w
+
Placebo IV q4w†
Zoledronic Acid 4 mg IV q4w†
+
Placebo SC q4w
Study 136[1]
Breast cancer
(N = 2049)
Study 103[2]
Prostate cancer
(N = 1904)
Study 244[3]
Other solid tumors/MM
(N = 1779)](https://image.slidesharecdn.com/managemaentofbonesecondaries-150330081013-conversion-gate01/85/Managemaent-of-bone-secondaries-33-320.jpg)

![Guidelines and Duration of Bone-
Targeted Therapy
ESMO
[1]
“The timing and optimal duration of bisphosphonate treatment are
unknown; benefit of duration beyond 2 yrs has not been demonstrated
. . . Long-term treatment seems wise due to ongoing risk of skeletal
events”
NCCN
[2]
“Optimal schedule and duration are unknown . . . Limited long-term
safety data indicating bisphosphonate treatment can continue beyond
2 yrs”
ASCO
[3]
“Until evidence of substantial decline (clinical judgment) in general
performance status”](https://image.slidesharecdn.com/managemaentofbonesecondaries-150330081013-conversion-gate01/85/Managemaent-of-bone-secondaries-36-320.jpg)