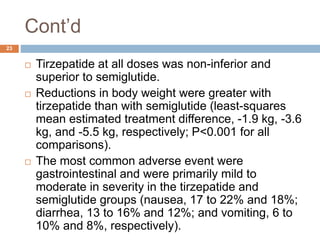
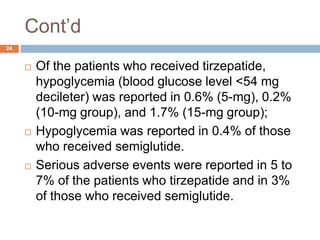
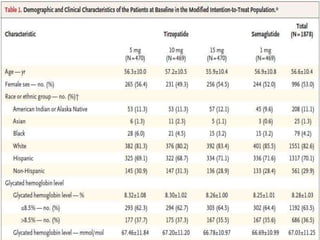
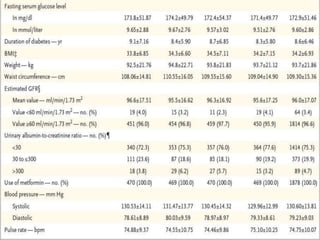
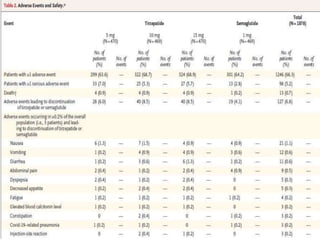
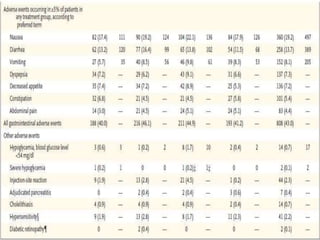
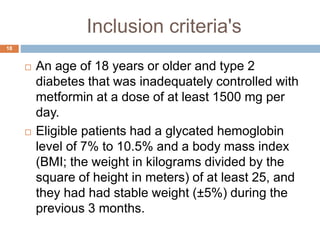
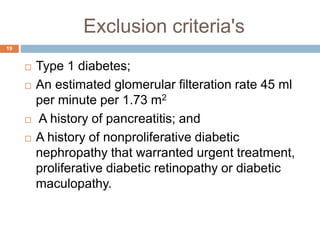
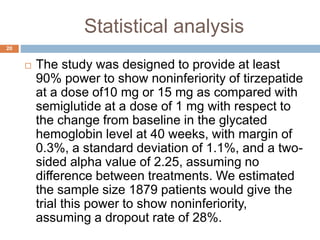
Tirzepatide was compared to semaglutide for treatment of type 2 diabetes in patients taking metformin in a 40-week randomized controlled trial. Tirzepatide at doses of 5mg, 10mg and 15mg resulted in greater reductions in HbA1c and body weight compared to semaglutide 1mg. Common side effects were mild to moderate gastrointestinal issues. Tirzepatide demonstrated superior glycemic control and weight loss over semaglutide and may allow more patients to achieve strict treatment targets while maintaining a low risk of hypoglycemia.











![Cont’d
12
The patients were randomly assigned in a
1:1:1:1 ratio to receive a once-weekly
subcutaneous injection of either tirzepatide (at
a dose of 5 mg, 10 mg, or 15 mg; the doses
were double blinded) or semiglutide (1 mg) for
a 40 week treatment period, followed by a 4-
week.
The patients were stratified at randomization
according to country and baseline glycated
hemoglobin level (≤8.5 % or >8.5%[≤69 or
>69mmol per mol]).](https://image.slidesharecdn.com/journalclubpresentation-220604034213-9e22d24f/85/Literature-Evaluation-pptx-12-320.jpg)

![The composite end point
16
A composite end point of a glycated
hemoglobin level of 6.5% or less with at least
10% weight l loss and without clinically
significant glycemia (blood glucose level, <54
mg per deciliter [3mmol per liter ] or severe
hypoglycemia events were also assessed.](https://image.slidesharecdn.com/journalclubpresentation-220604034213-9e22d24f/85/Literature-Evaluation-pptx-16-320.jpg)

![Cont’d
22
The estimated mean from baseline in the glycated
hemoglobin level was -2.01percentage points, -
2.24 percentage points, and -2.30 percentage with
5 mg, 10 mg and 15 mg of tirzepatide respectively
and -1.86 percentage points with semiglutide; the
estimated differences differences between the 5-
mg, 10-mg, and 15-mg tirzepatide groups and the
semiglutide group were -0.15 percentage points
(95% confidence interval [CI], -0.28 to -0.03;
P=0.02), -0.39 percentage points (95% CI, -0.51to
-0.26; P<0.001), and -0.45 percentage points
(95% CI, -0.57 to -0.32; P<0.001), respectively.](https://image.slidesharecdn.com/journalclubpresentation-220604034213-9e22d24f/85/Literature-Evaluation-pptx-22-320.jpg)