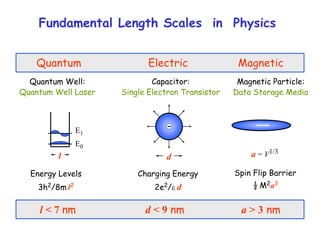
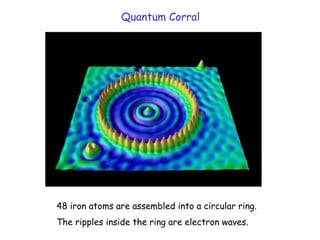
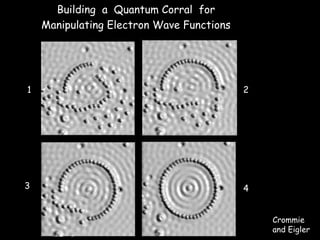
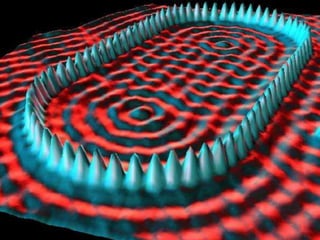
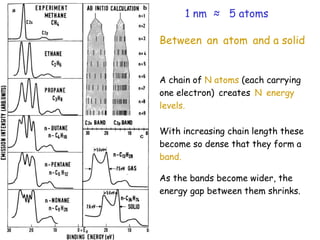
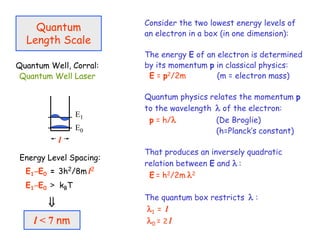
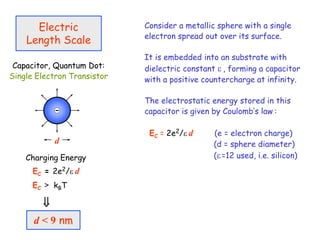
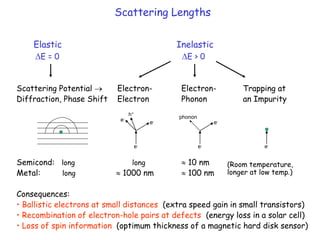
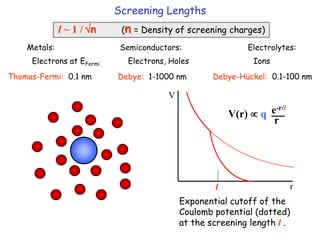
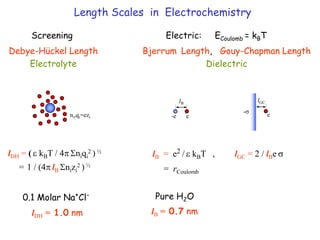
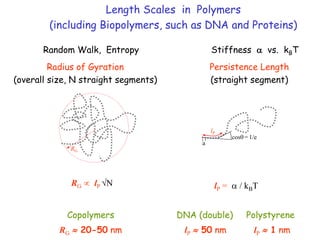
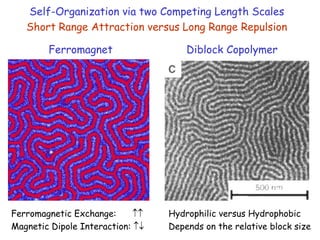
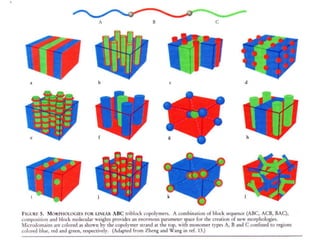
The document discusses various length scales that are important in physics, chemistry, and biology. It provides examples of fundamental length scales in quantum physics where quantum effects become prominent below 7 nanometers. In electric phenomena, the charging energy of a single electron becomes comparable to thermal energy below 9 nanometers. For magnetic structures, the energy barrier for flipping the spin of a magnetic particle must be larger than thermal energy, which requires a size above 3 nanometers. The document also discusses important length scales for polymers, electrolytes, and self-organization driven by competing short-range attraction and long-range repulsion forces between different blocks or particles.