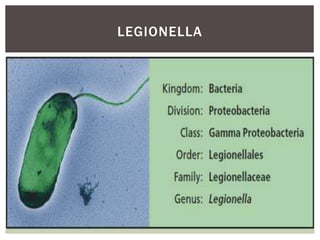
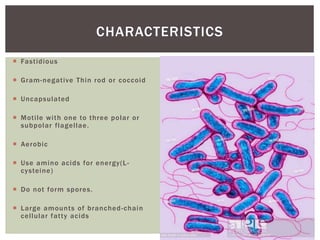
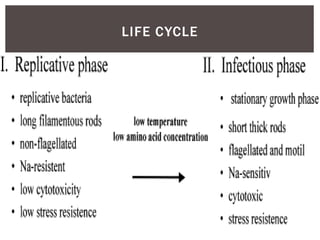
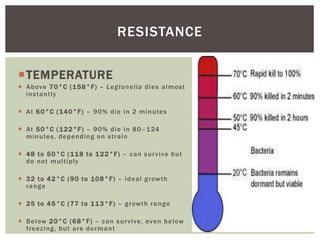
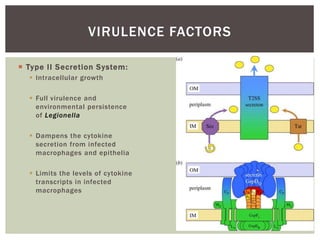
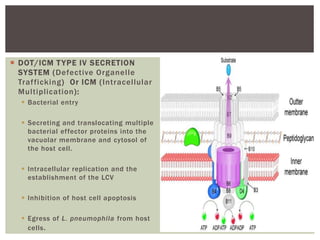
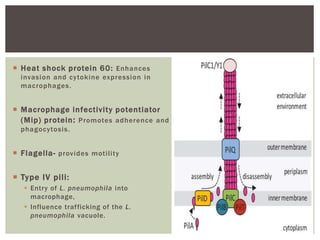
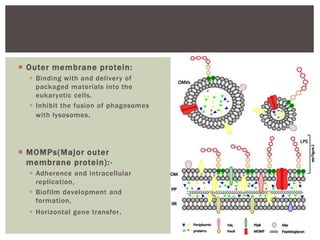
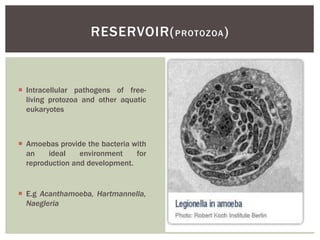
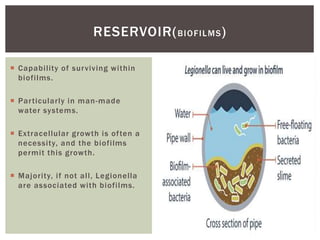
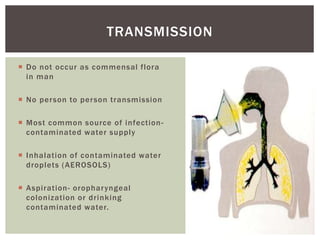
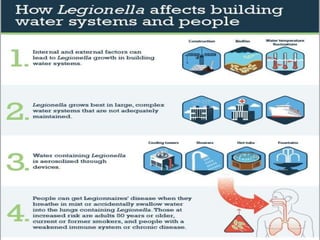
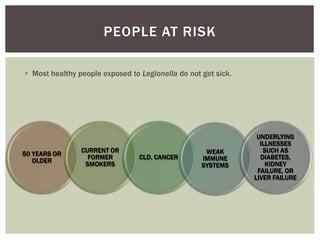
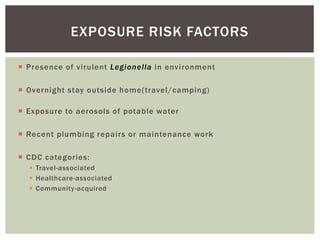
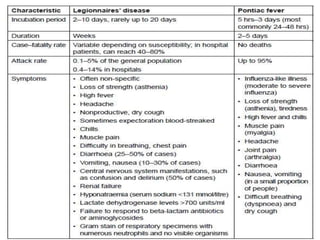
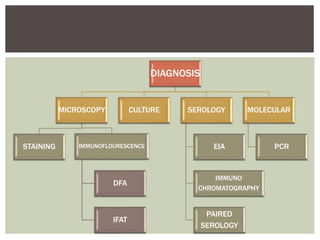
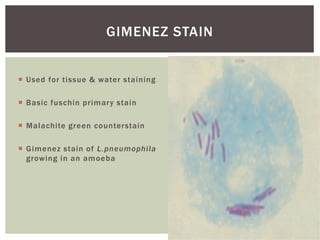
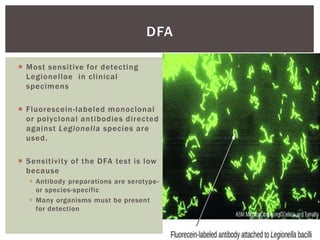
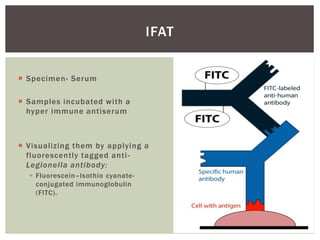
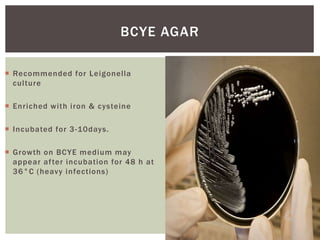
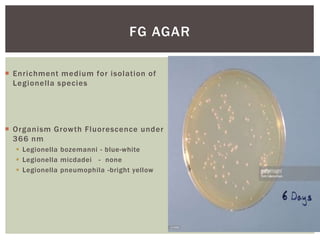
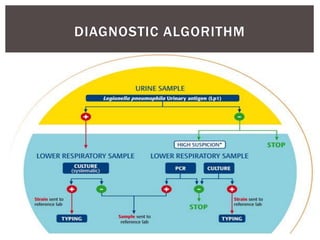
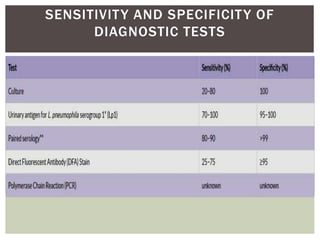
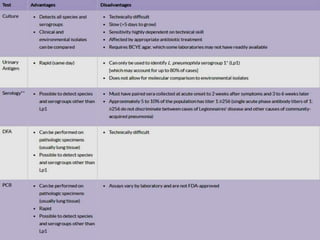
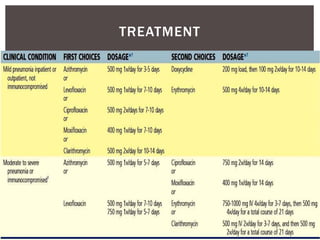
This document provides information on Legionella, including its classification, species that cause human infections, reservoirs, transmission, clinical manifestations, diagnosis and treatment. Legionella is a genus of bacteria that can cause Legionnaires' disease or Pontiac fever in humans. L. pneumophila serogroup 1 is responsible for the majority of infections. Legionella is commonly found in water systems and can infect humans when contaminated water droplets are inhaled. Diagnosis involves urine antigen testing, culture, staining and PCR. Treatment involves antibiotics such as macrolides or fluoroquinolones. Preventing Legionella growth in water systems is key to preventing infections.