Introduction,
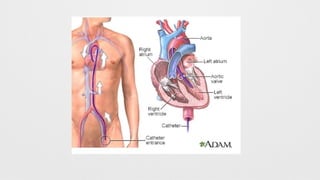
Left Heart Catheterization,
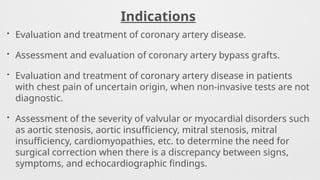
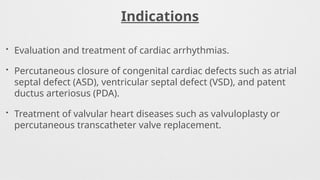
Indications,
Contraindications,
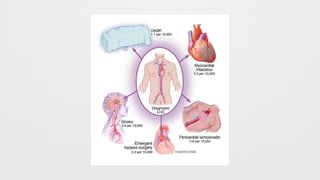
Complications,
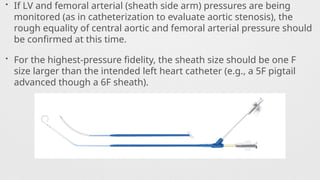
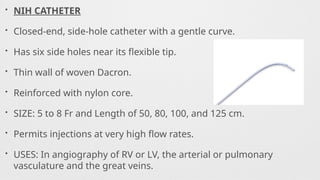
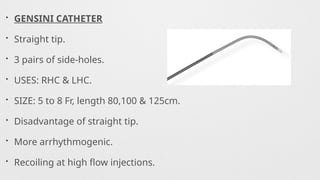
Catheters Used,
Procedure,
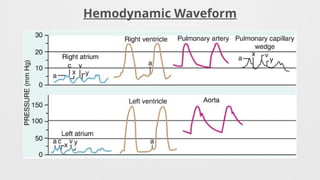
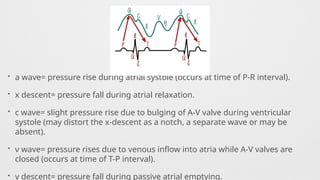
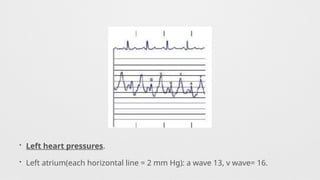
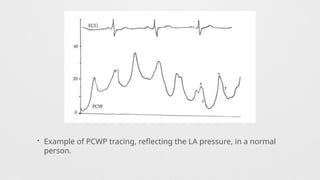
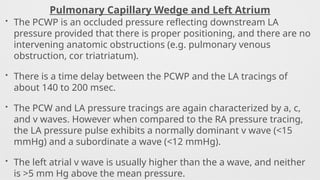
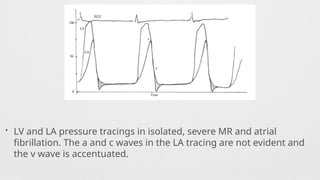
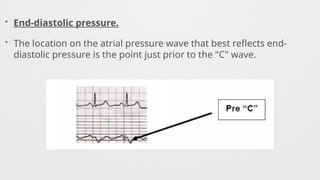
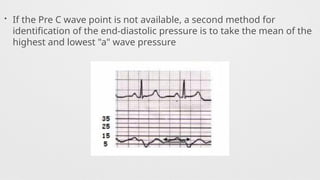
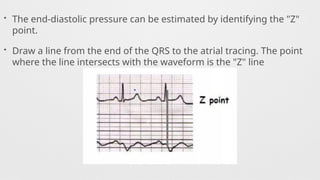
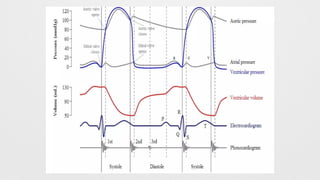
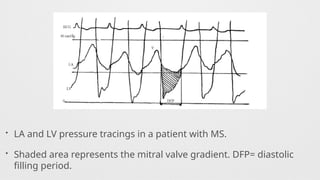
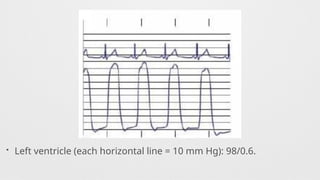
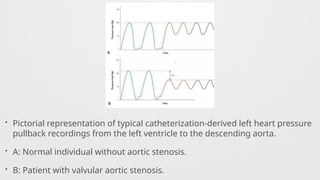
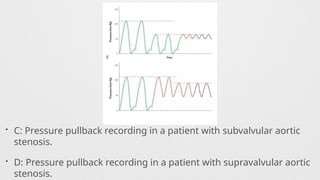
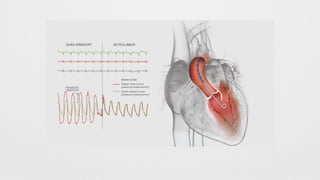
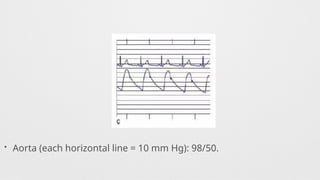
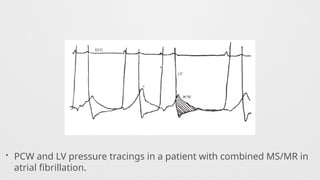
Hemodynamics,
Hemodynamics data,
Shunt Calculation,
Oxymetry,
Shunt Quantification,
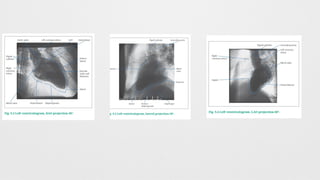
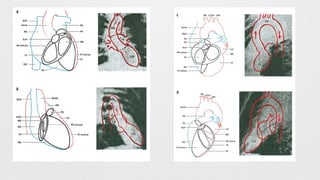
Left Ventriculogram,
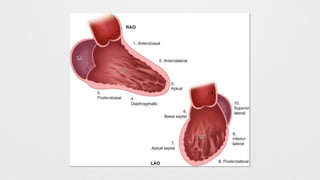
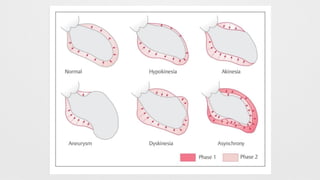
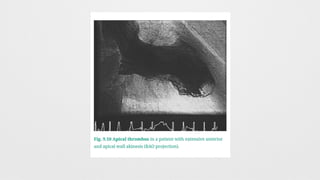
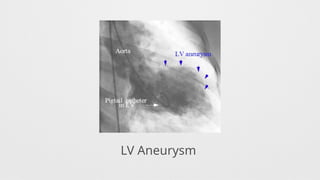
LV Wall motion abnormalities,
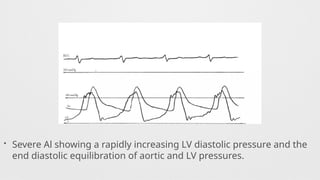
Angiographic Aortic Regurgitation,
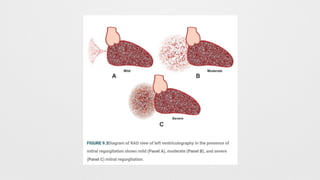
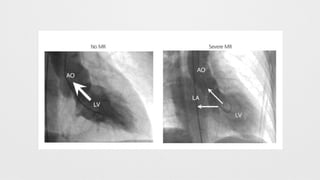
Angiographic Mitral Regurgitation