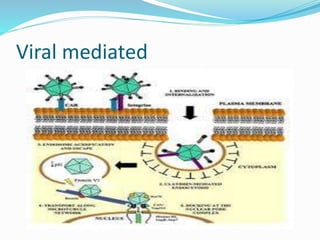
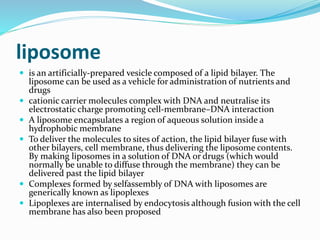
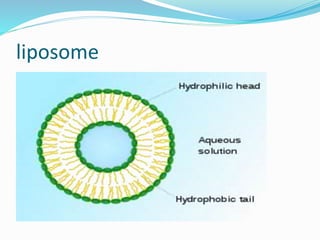
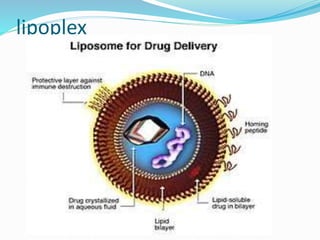
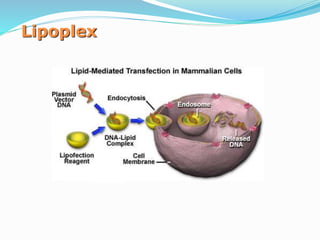
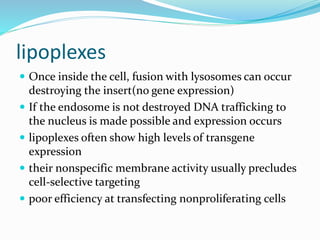
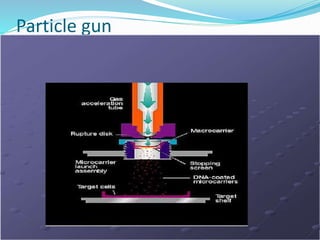
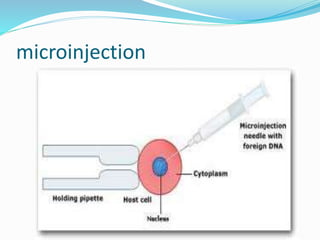
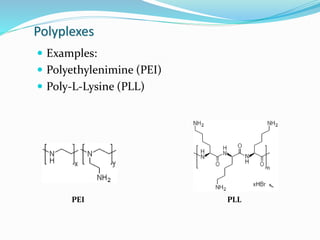
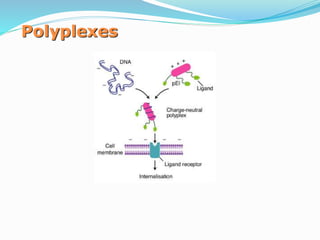
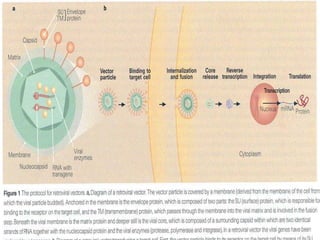
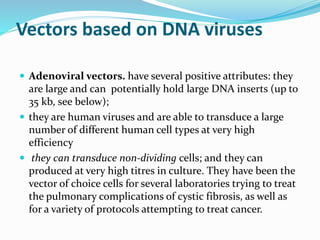
This document discusses various methods of gene transfer, including viral and non-viral methods. Viral methods use recombinant viruses like retroviruses and adenoviruses to insert genes into host cells. Non-viral methods include physical techniques like electroporation and gene guns that force DNA into cells, as well as chemical methods using lipids, polymers, or proteins to transport DNA into cells. Both methods have advantages and disadvantages related to efficiency, safety, and target specificity.