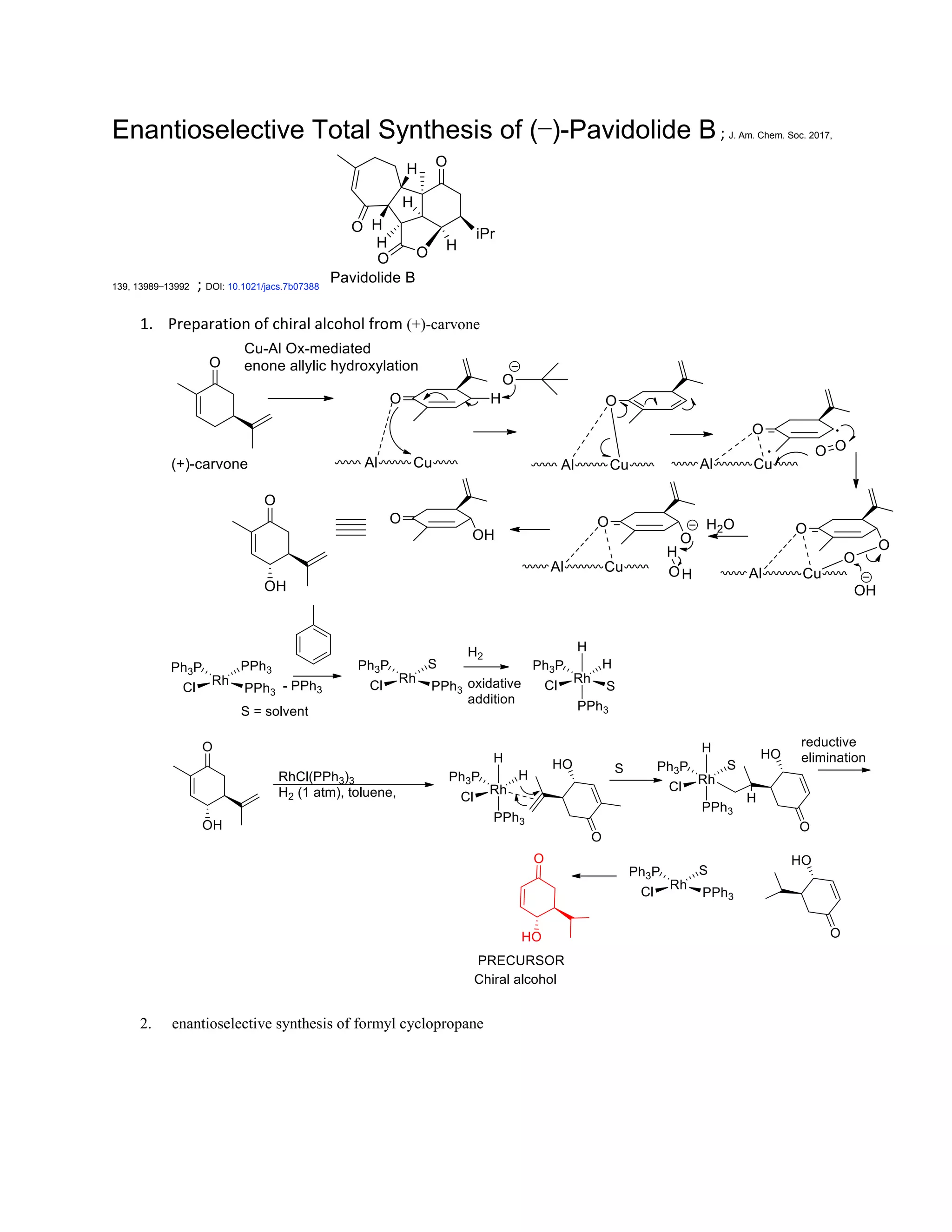
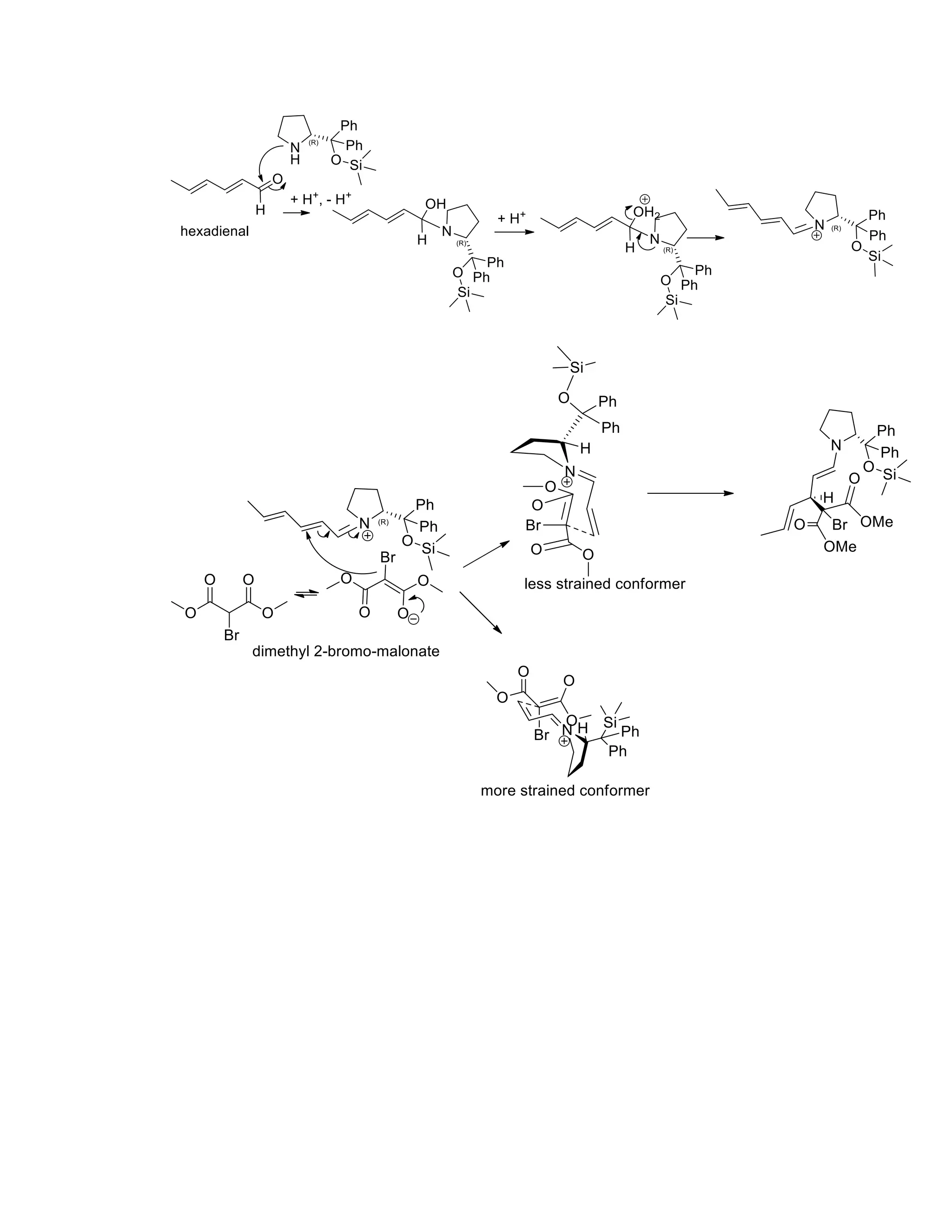
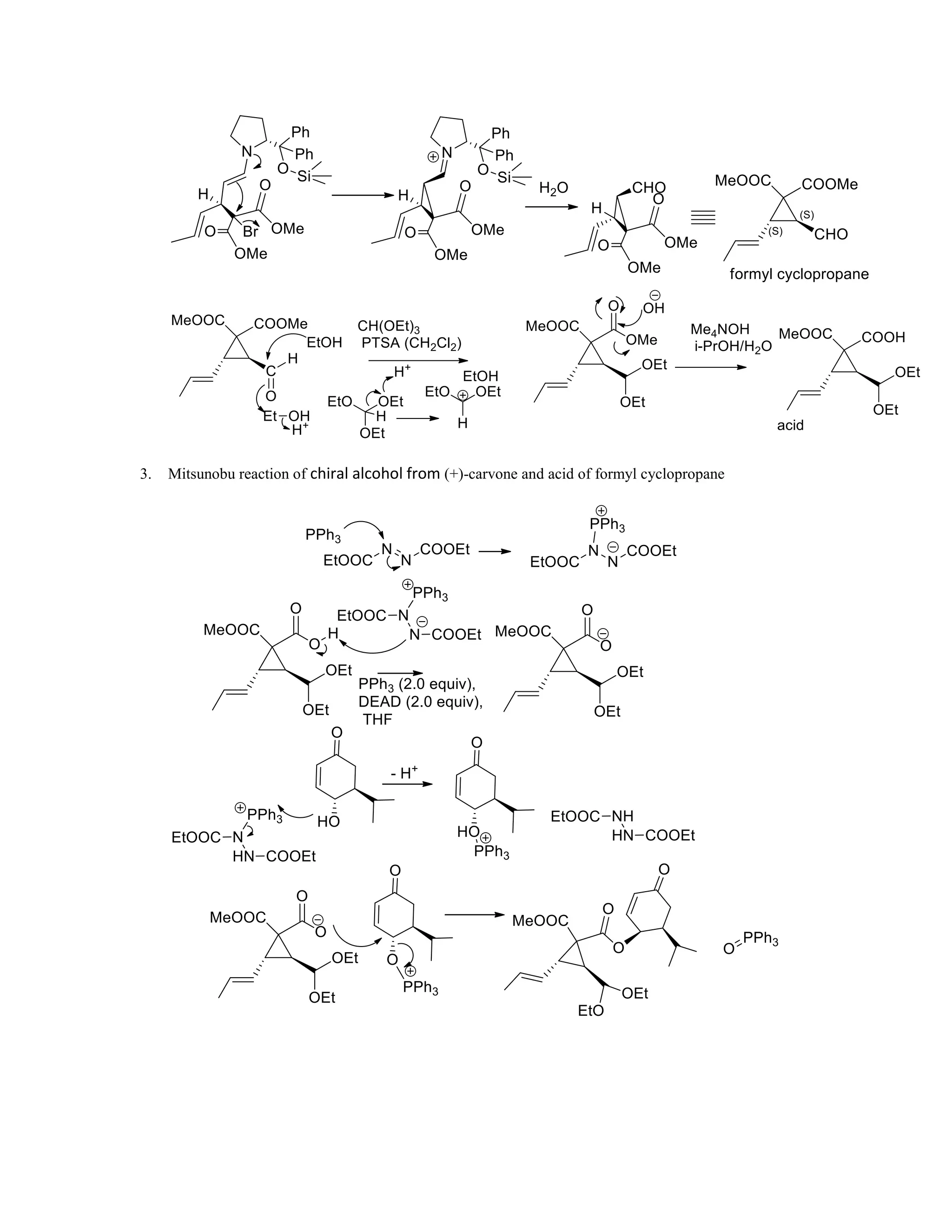
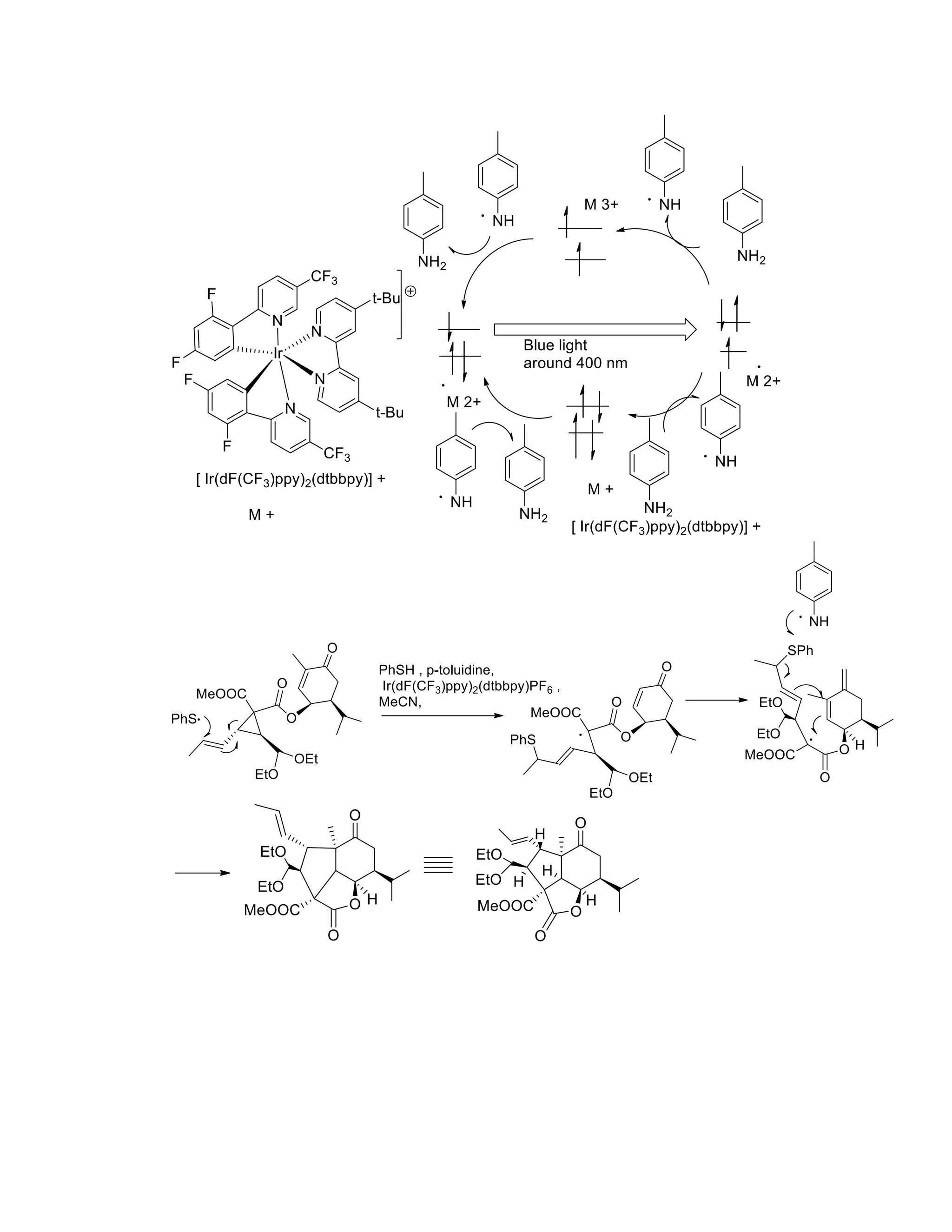
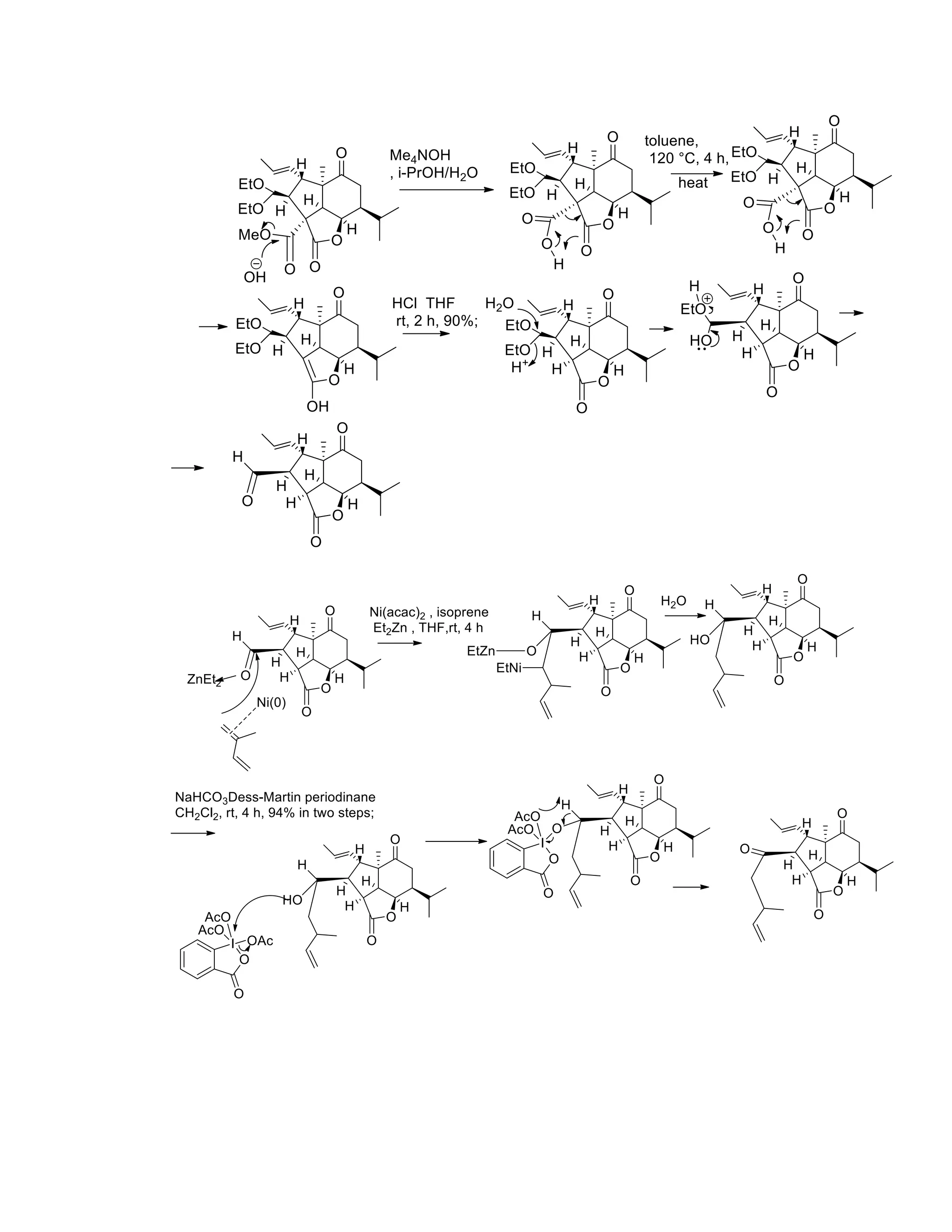
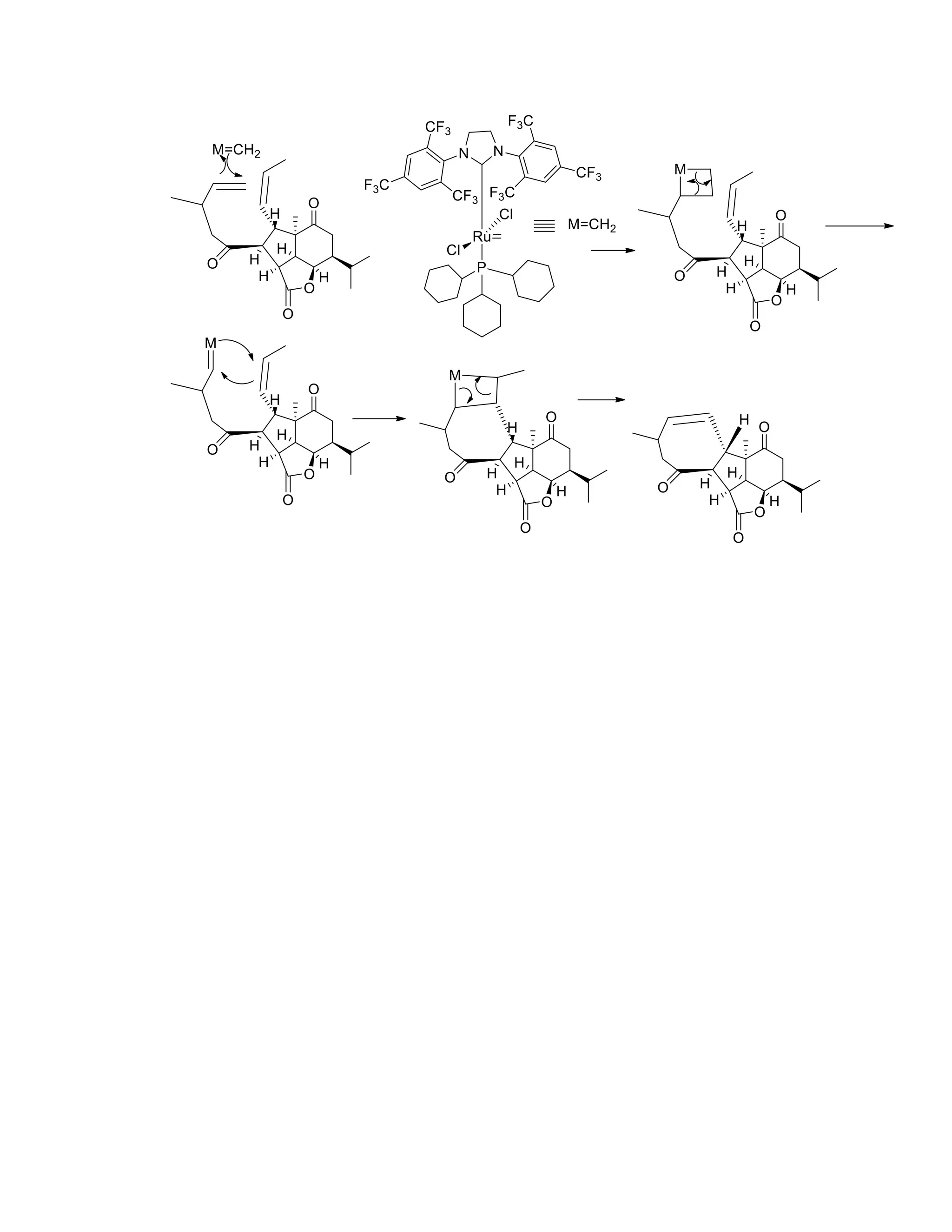
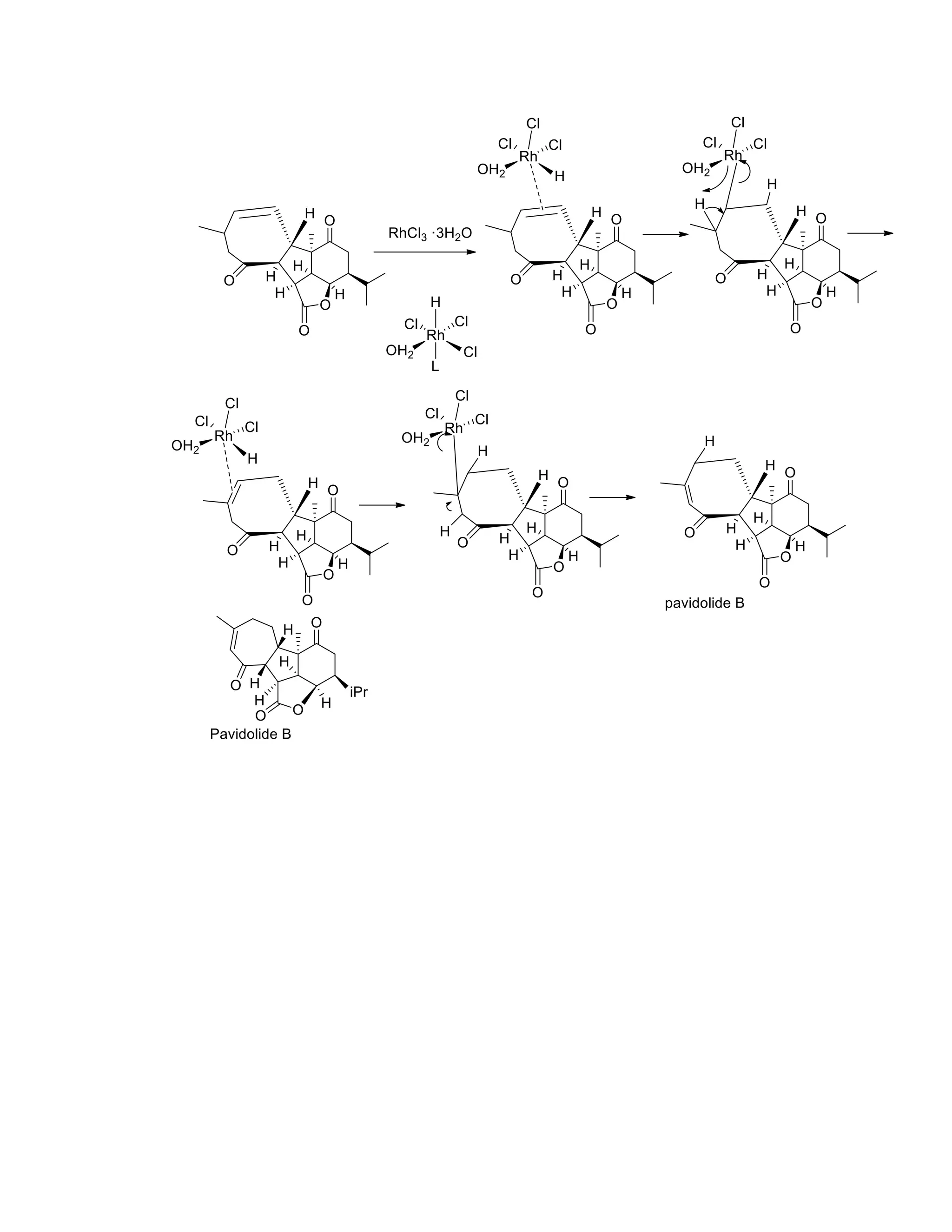
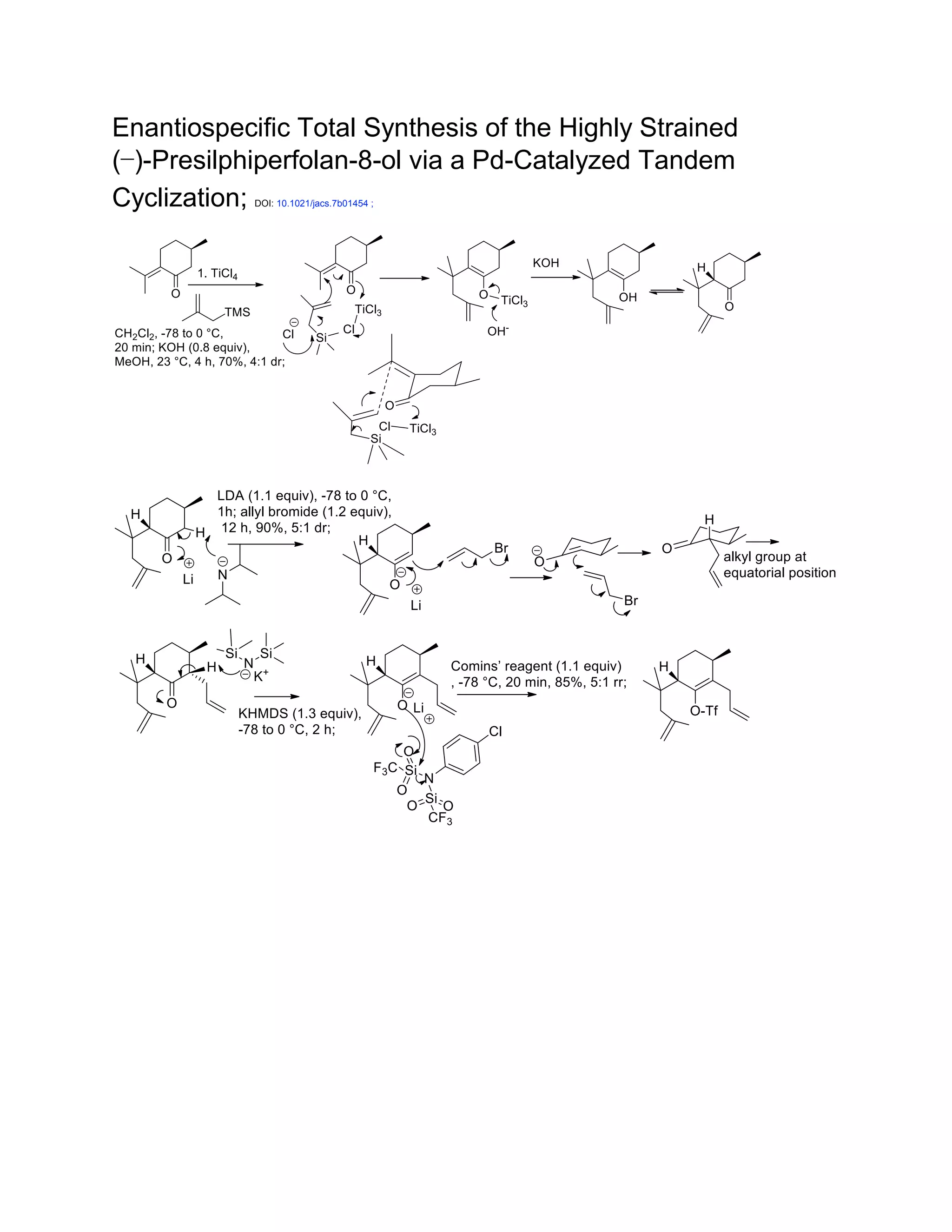
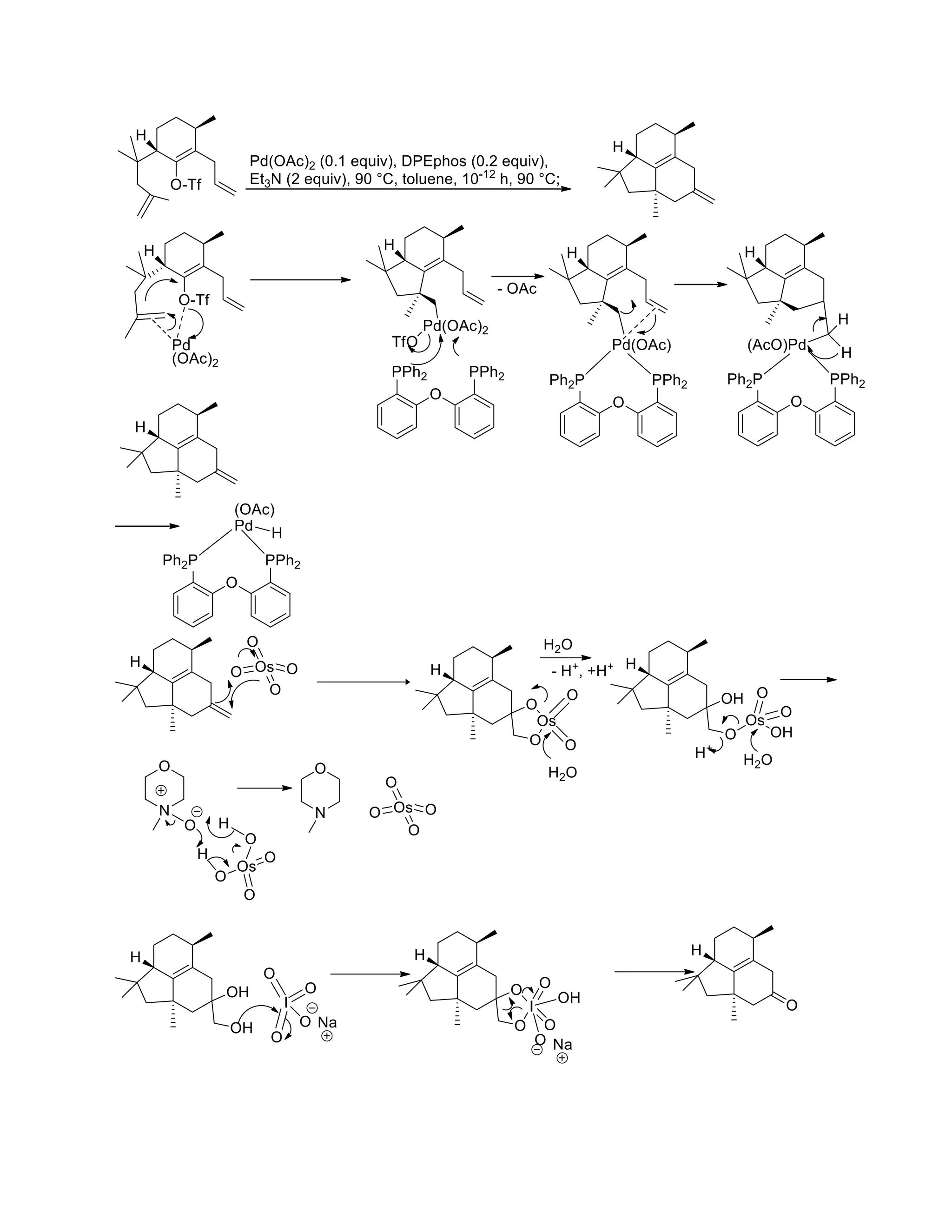
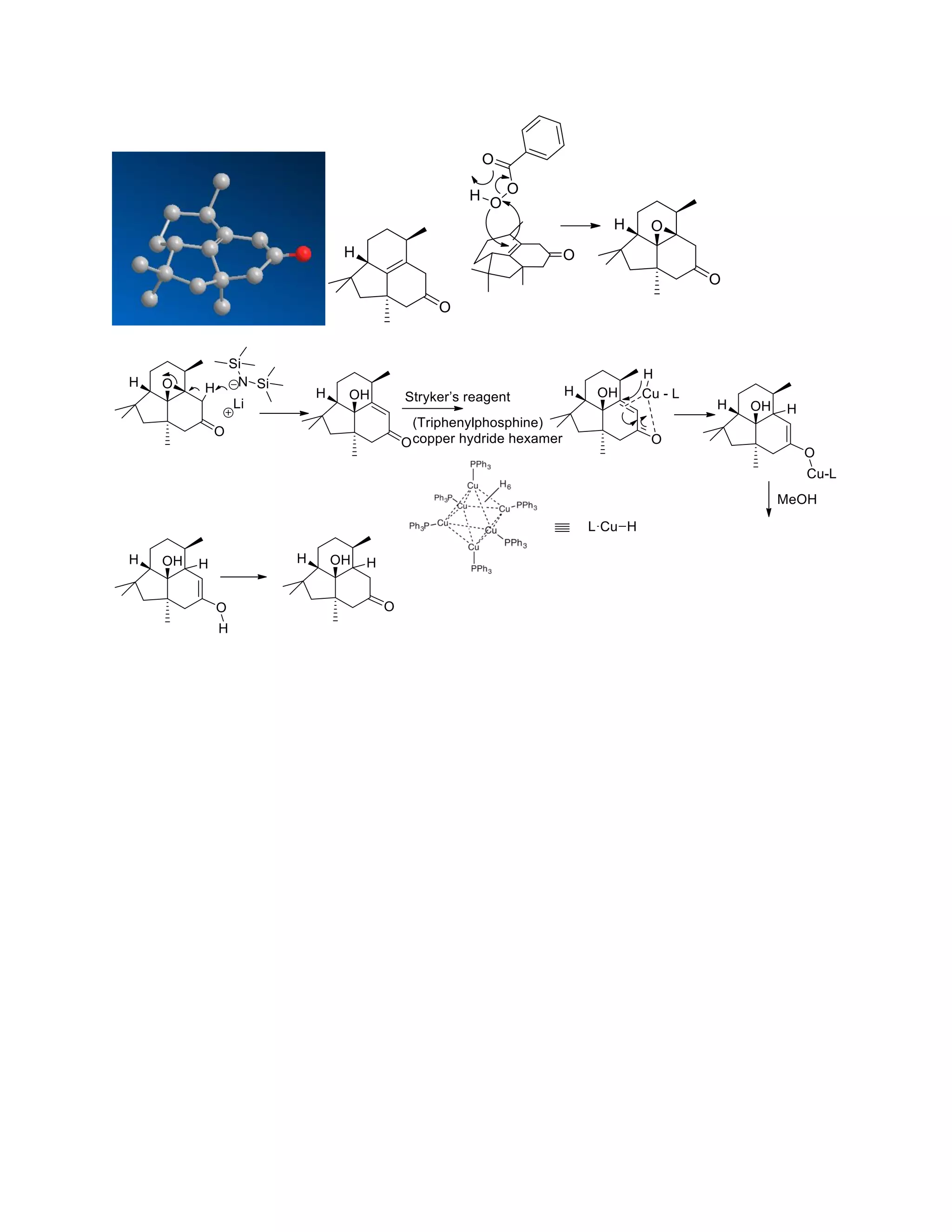
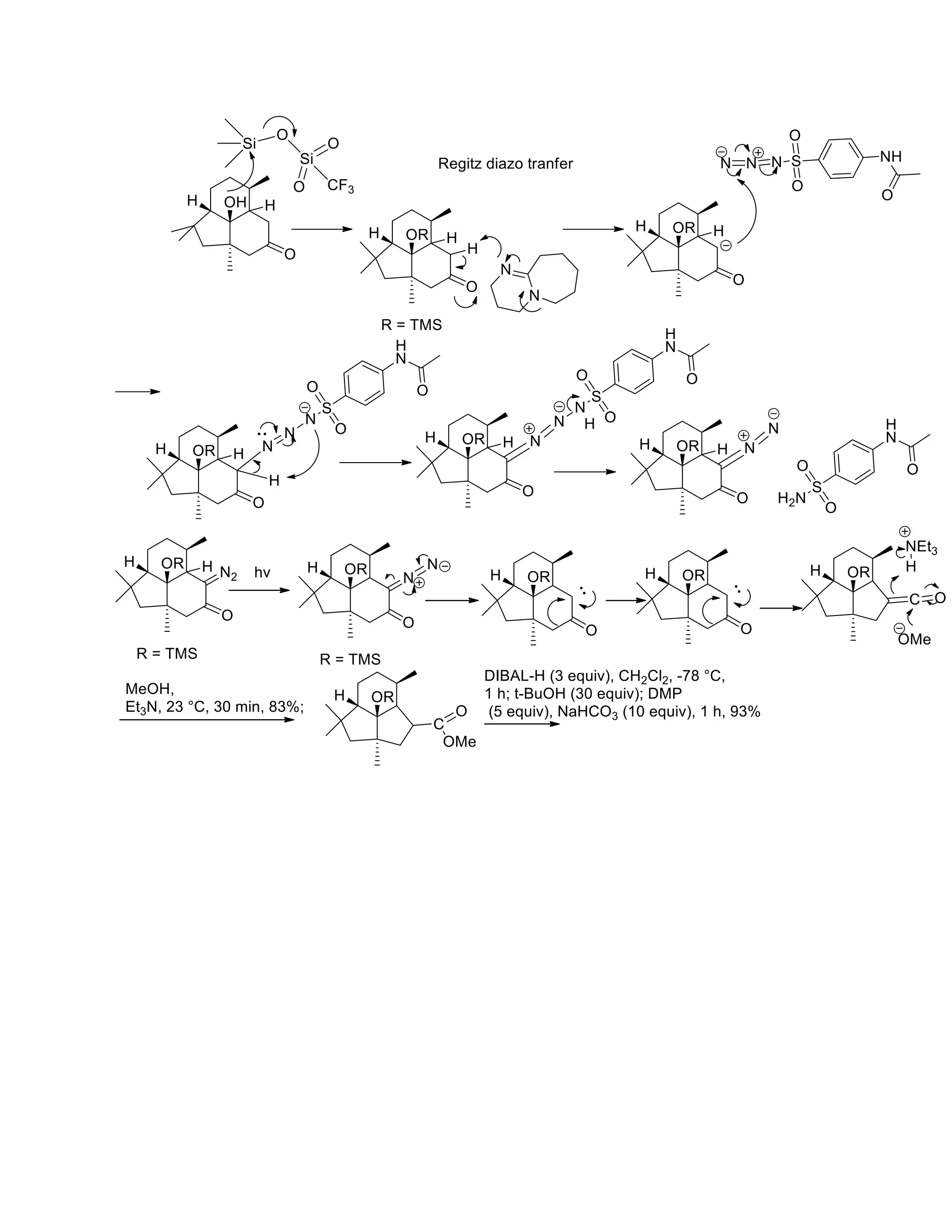
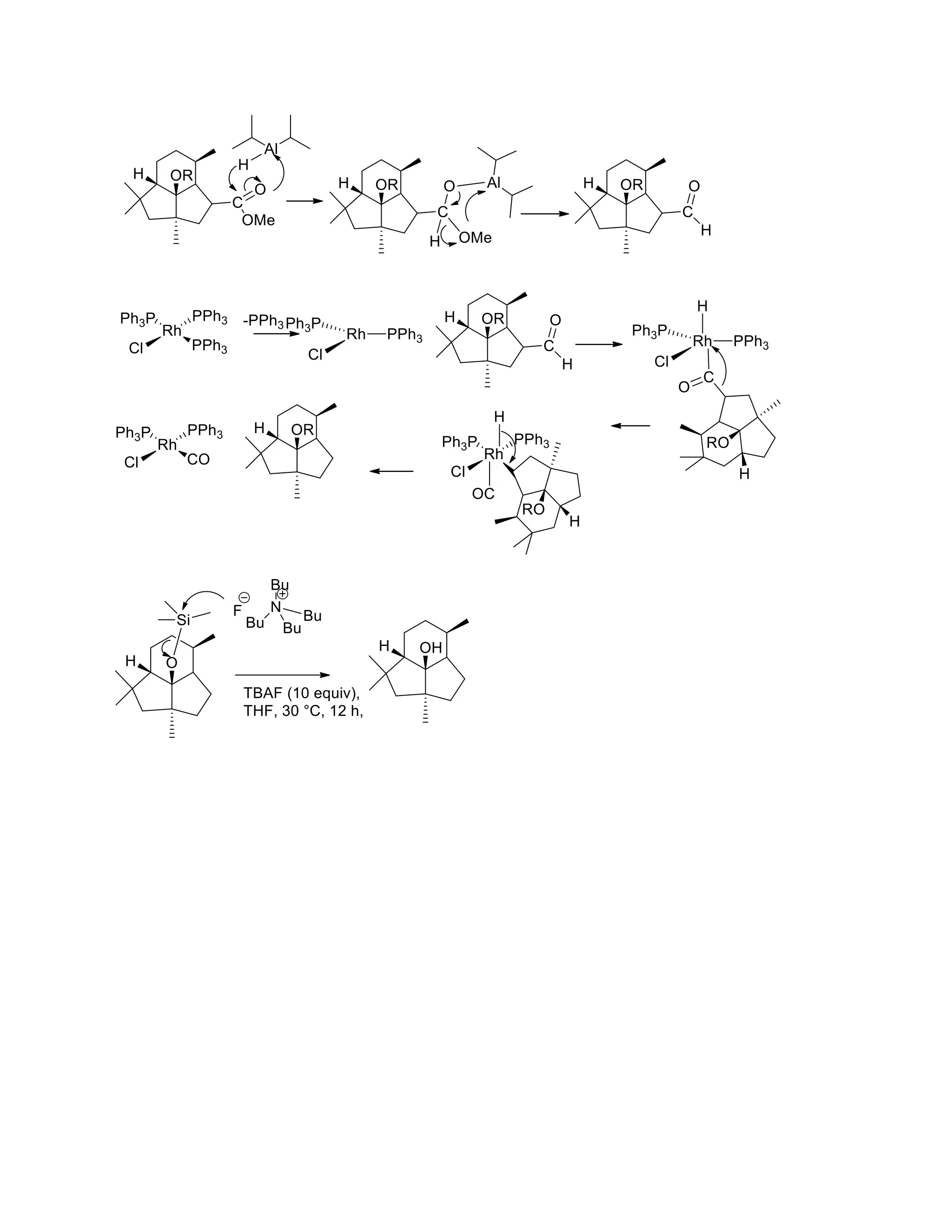
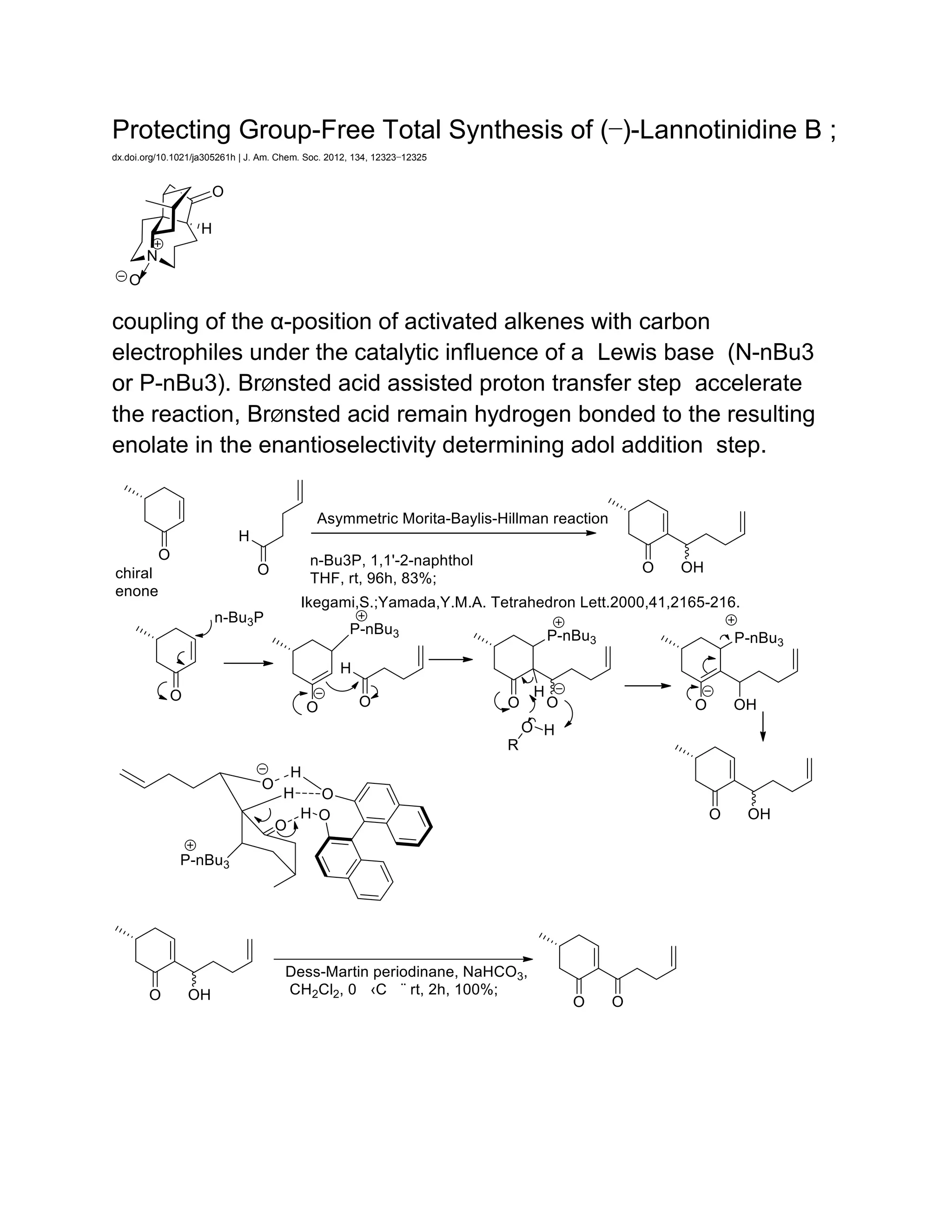
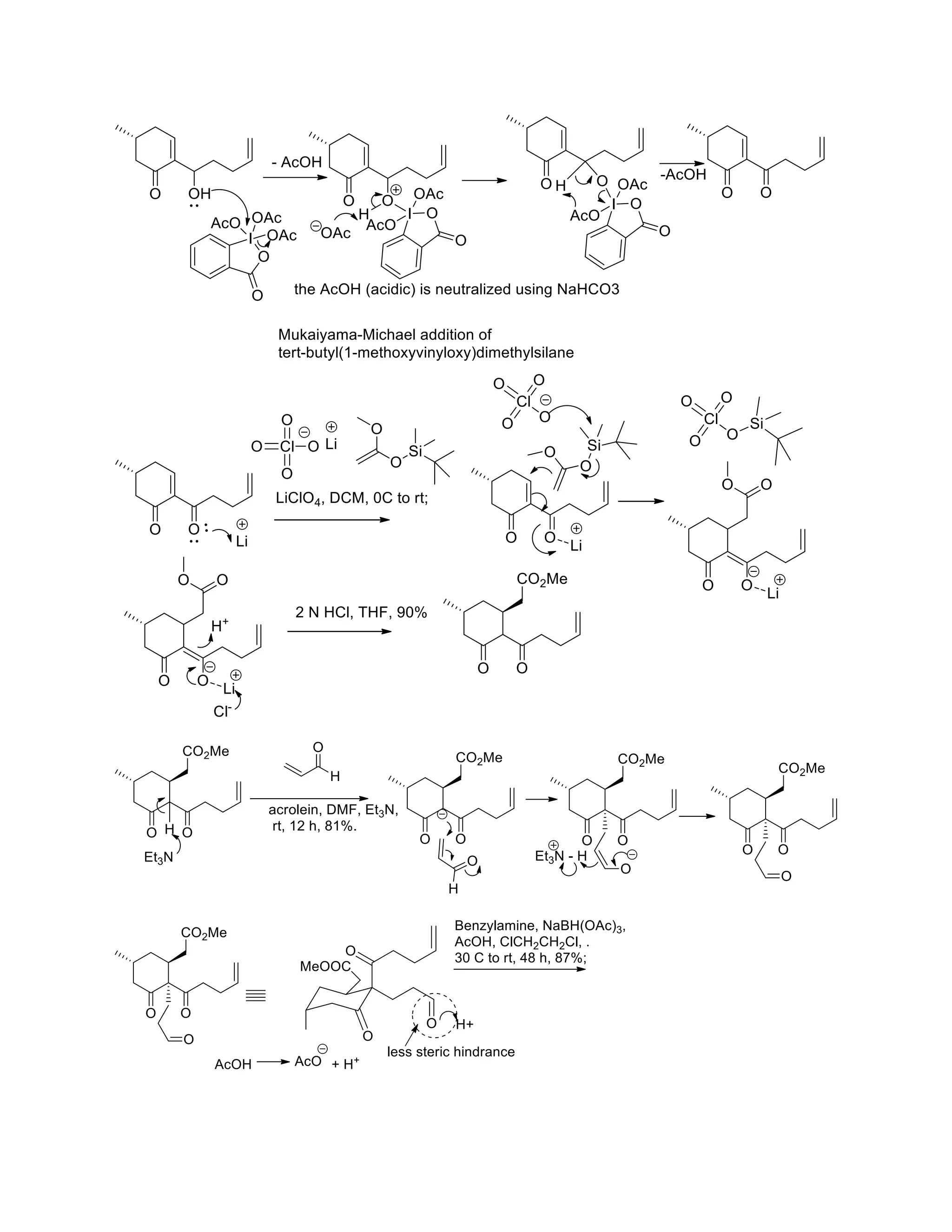
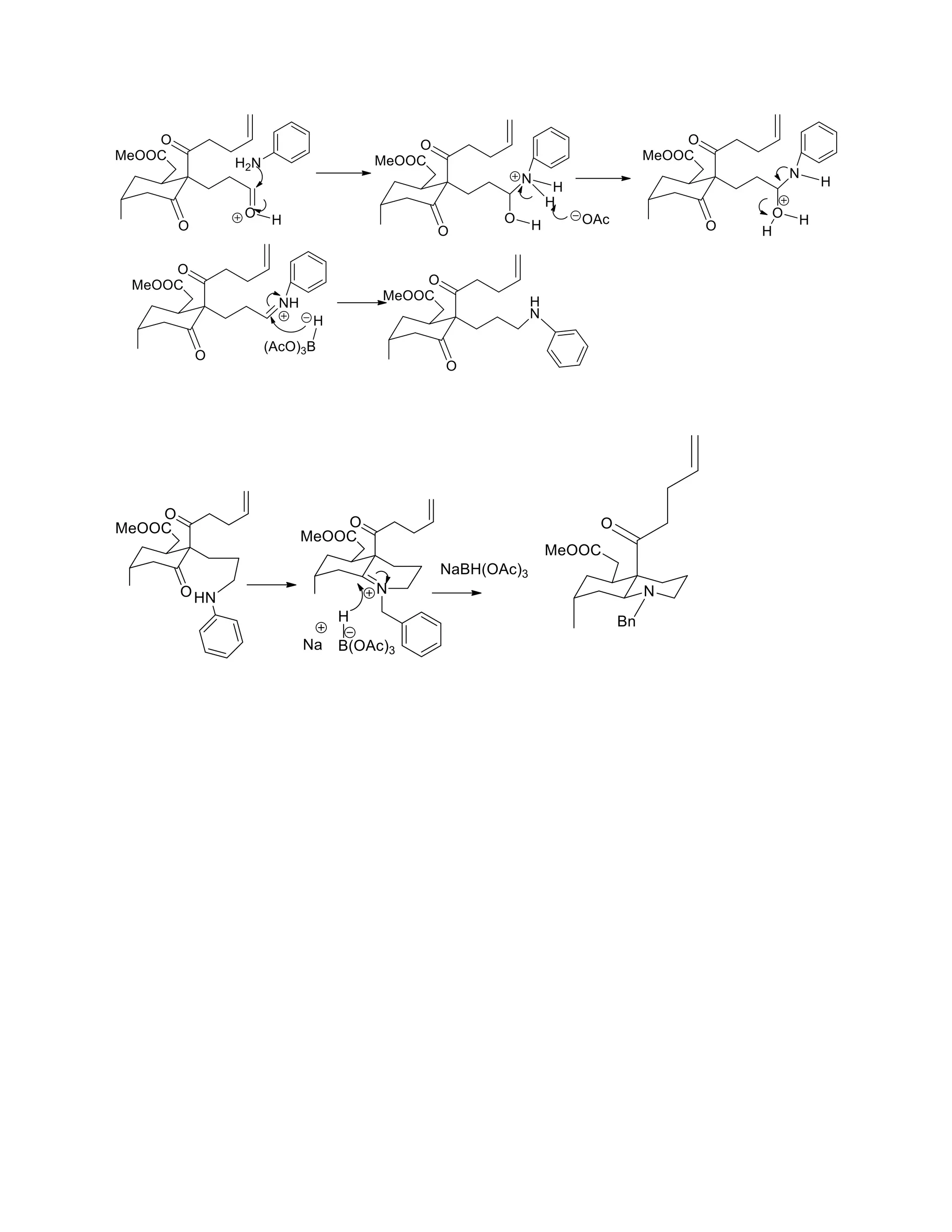
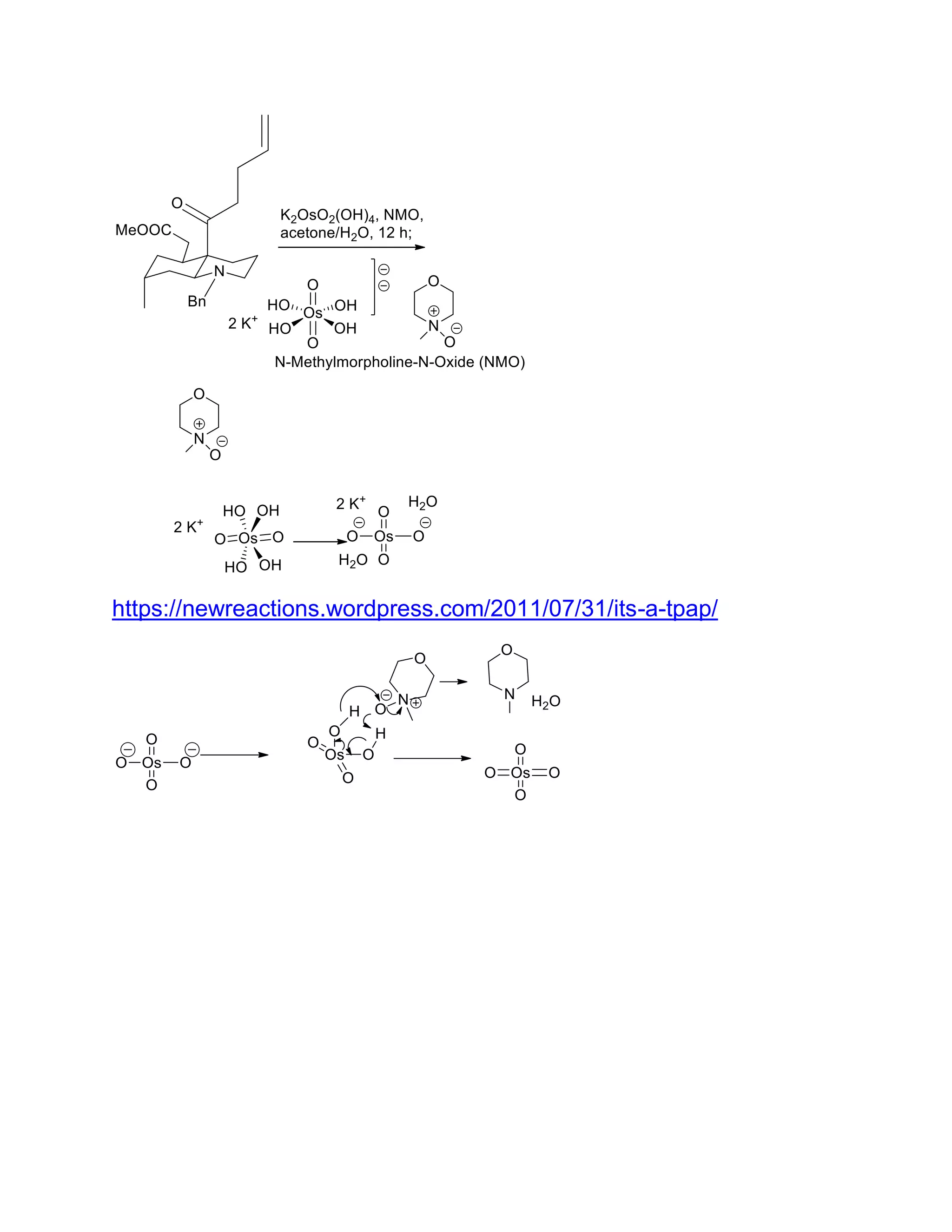
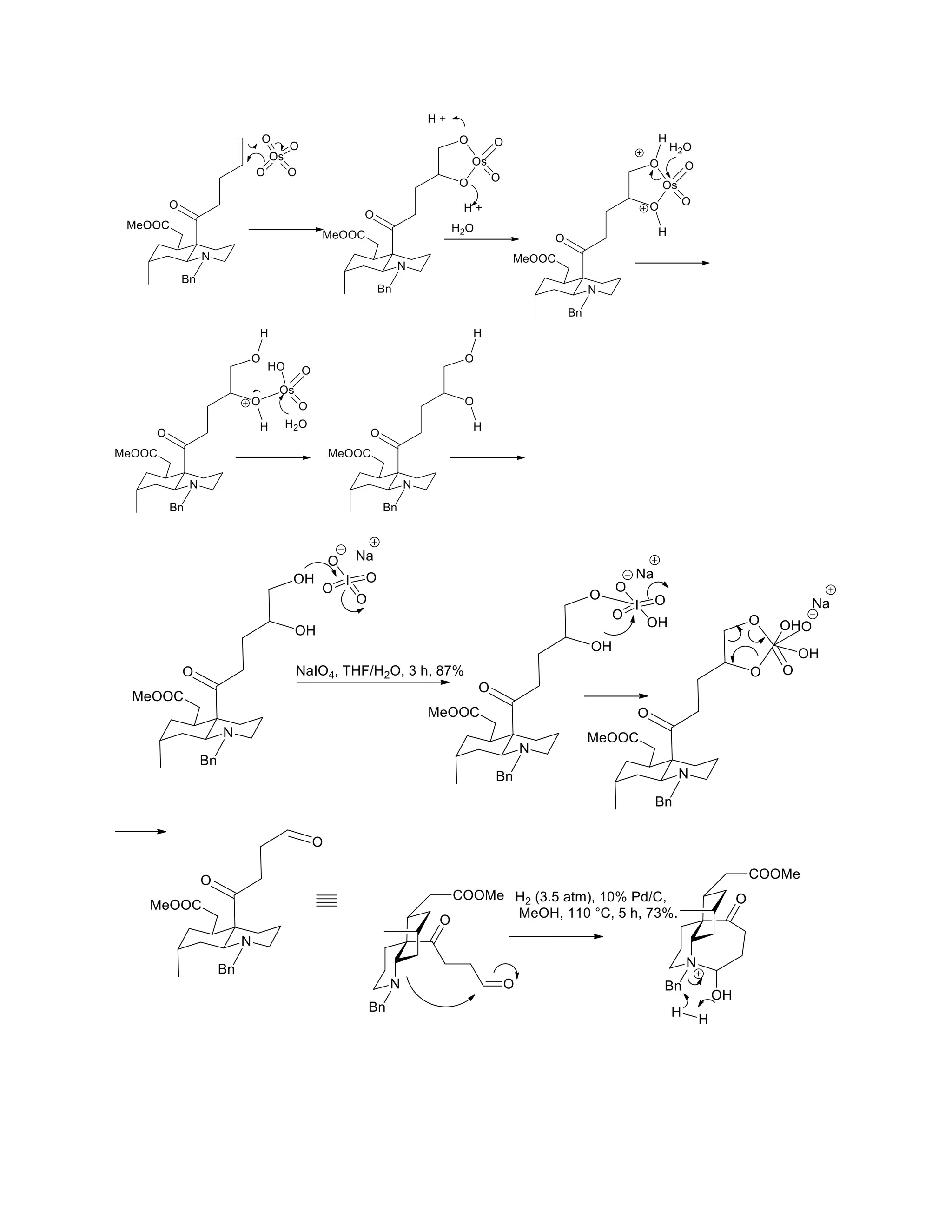
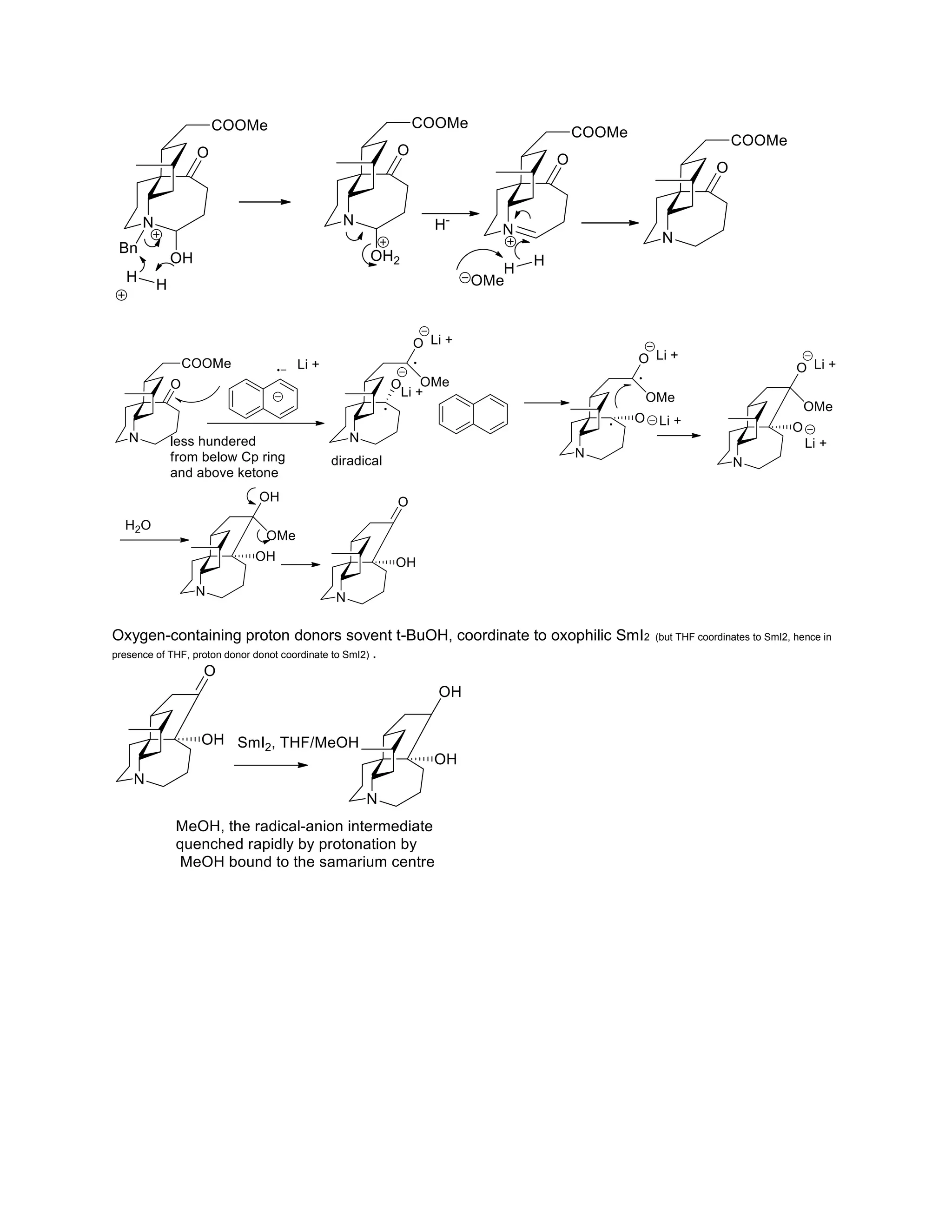
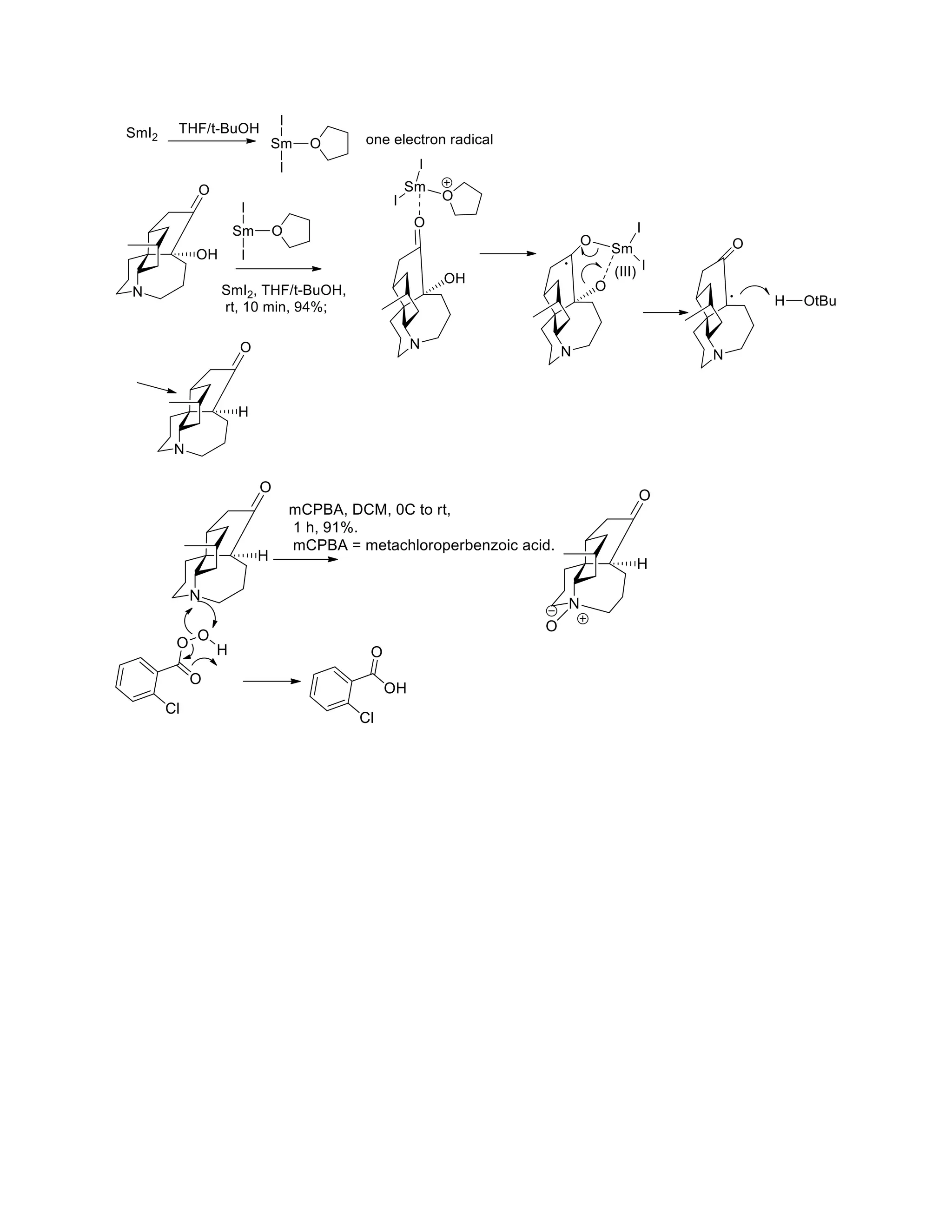
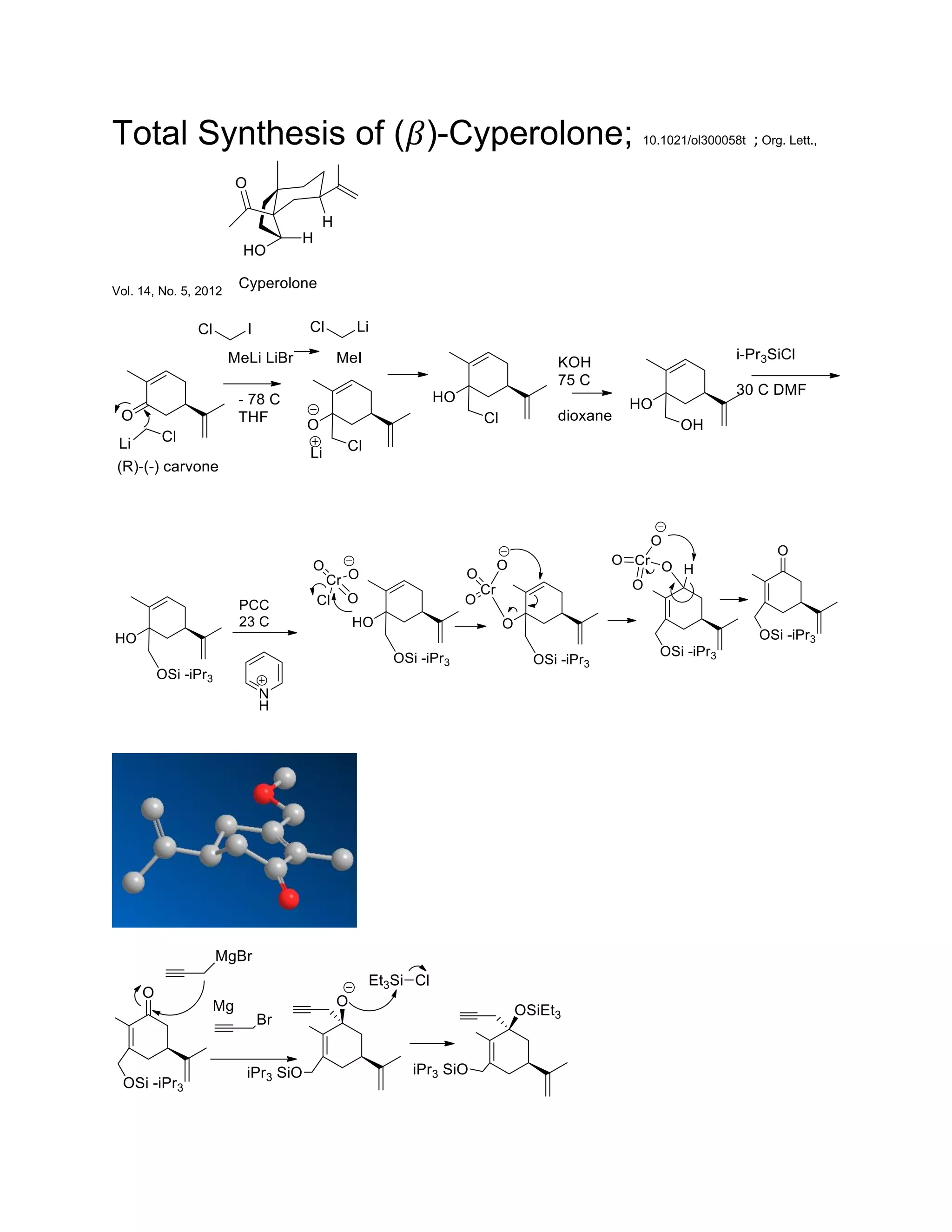
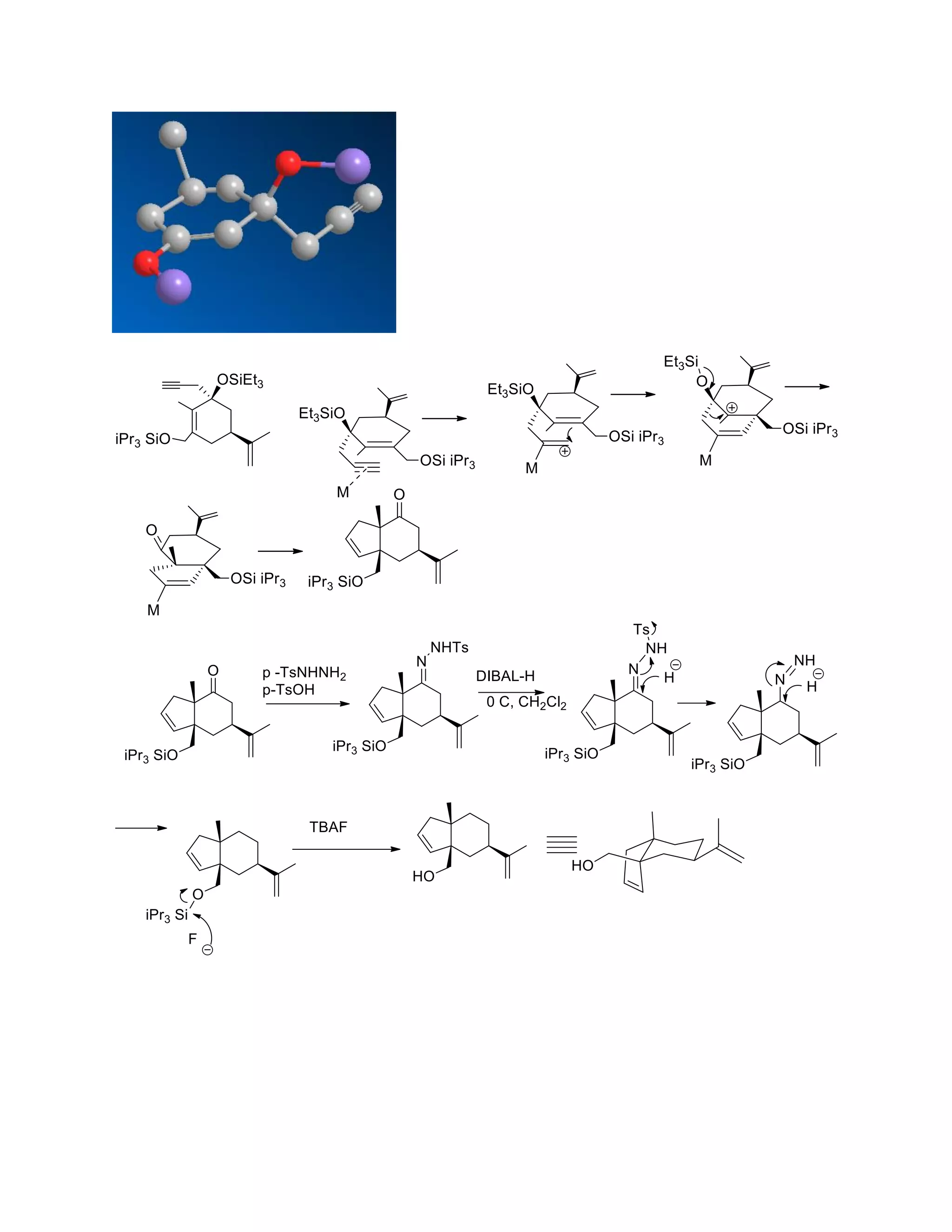
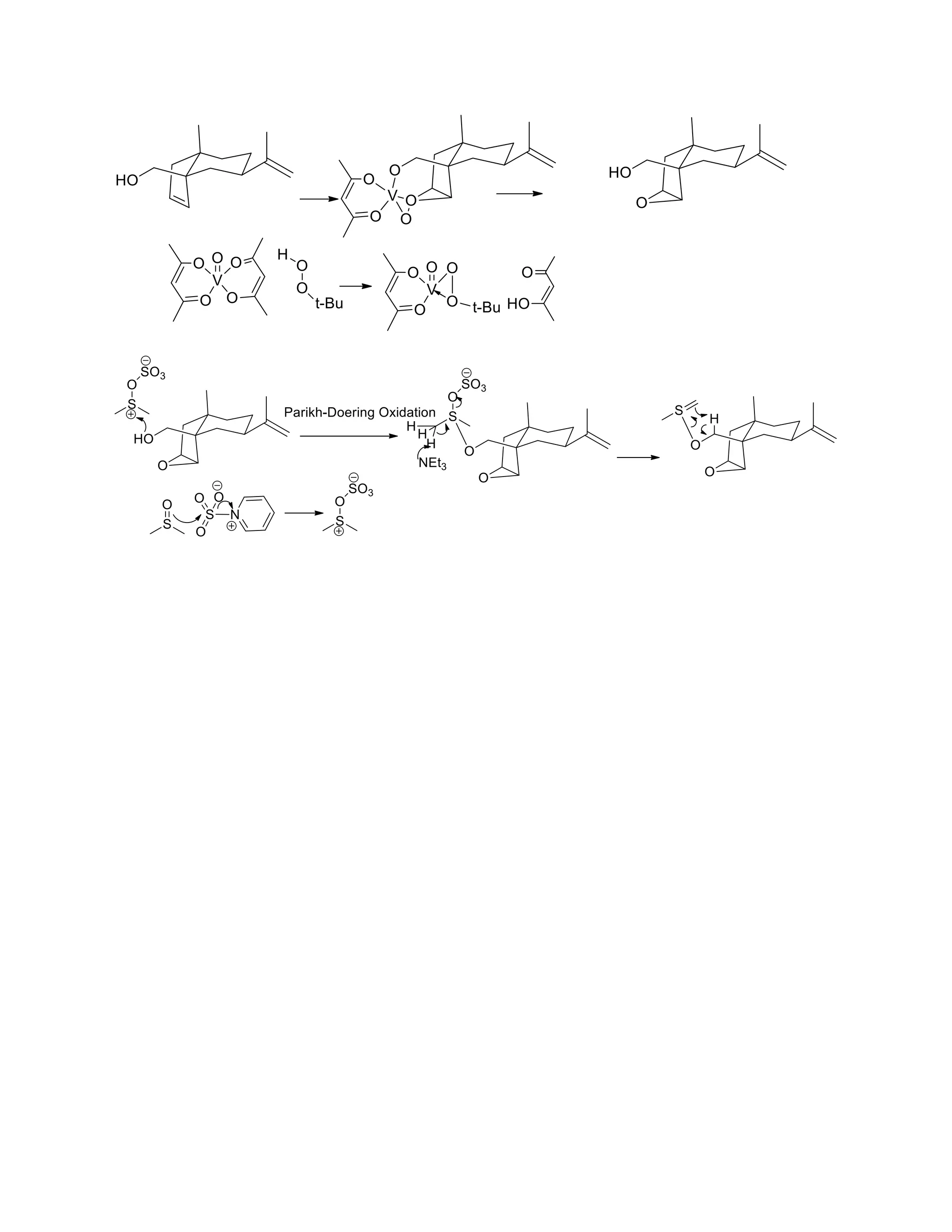
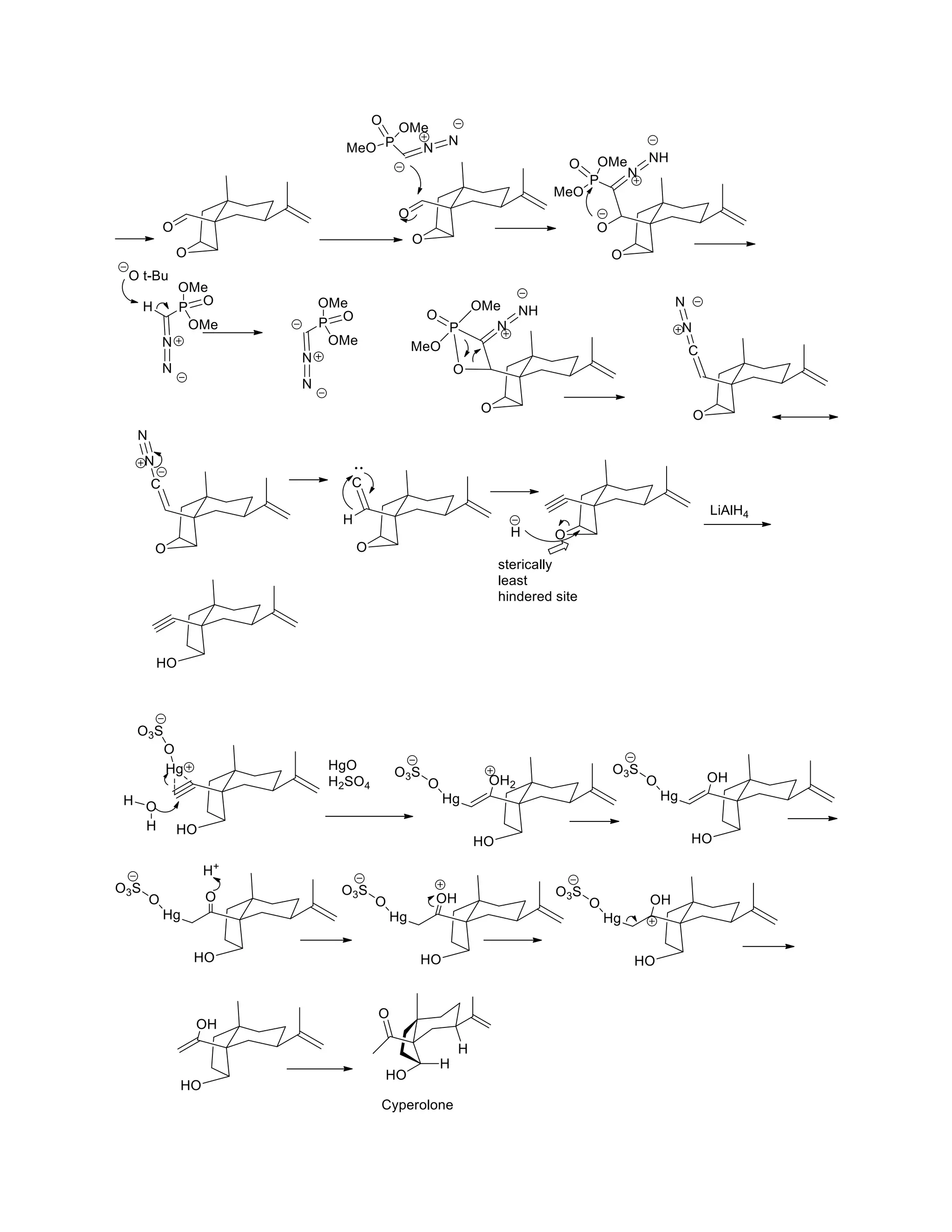
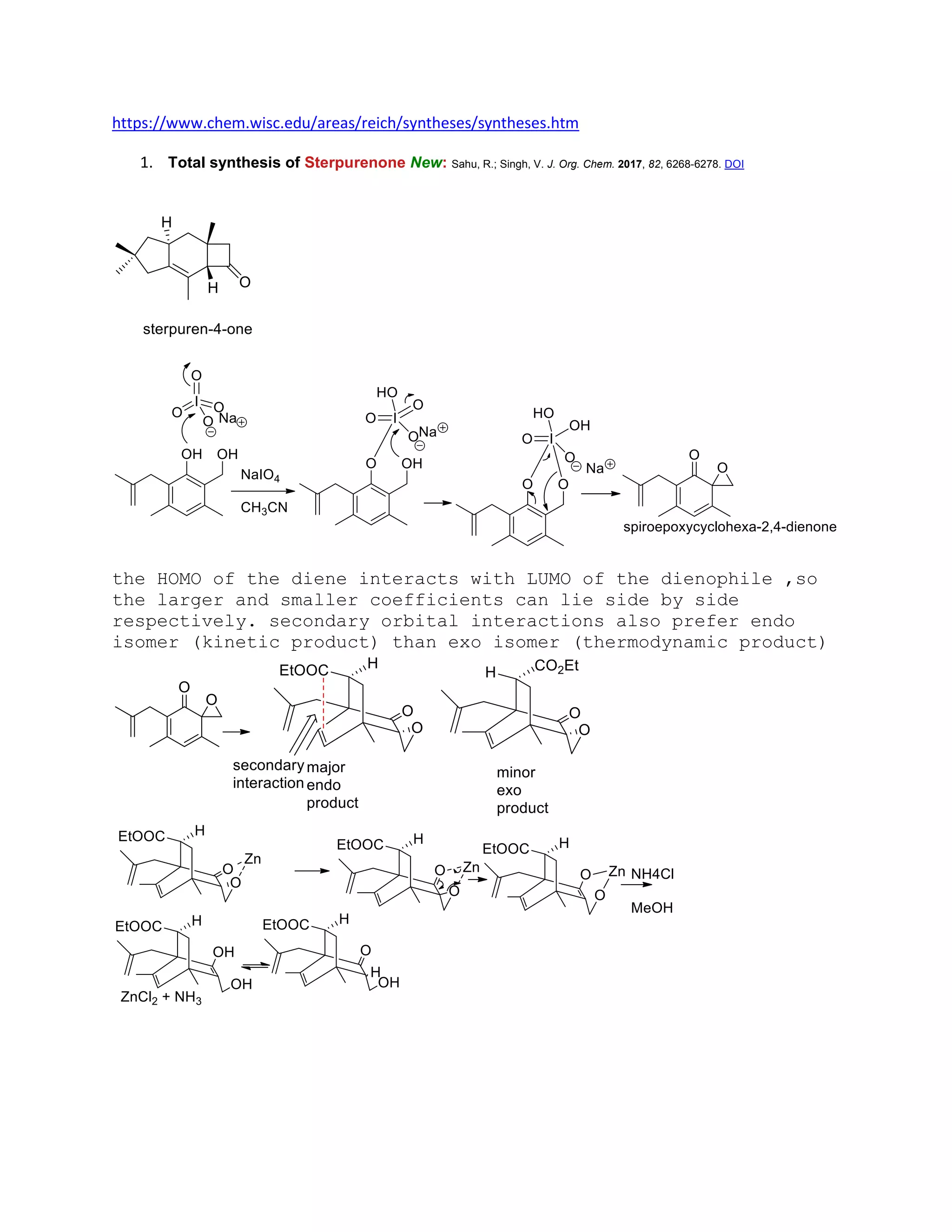
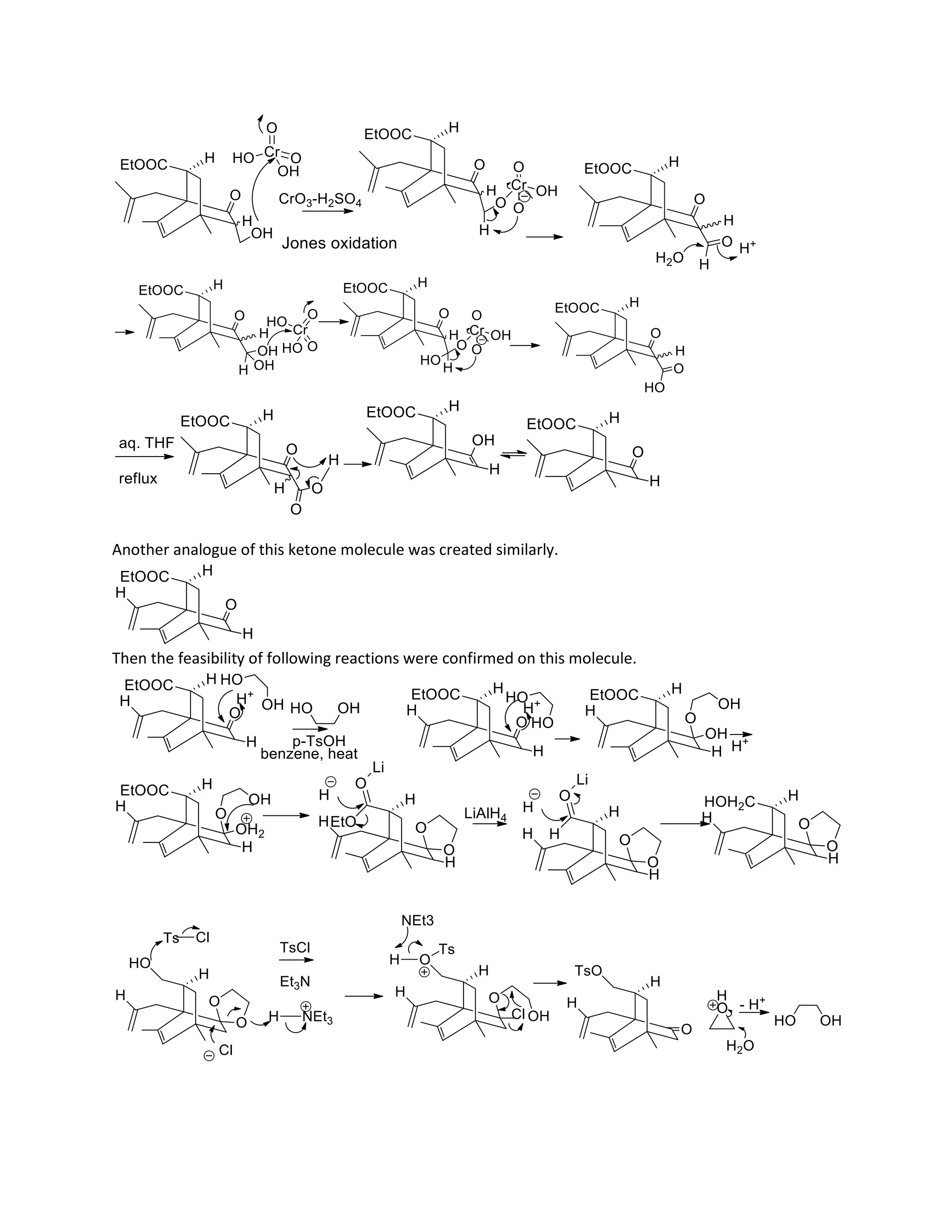
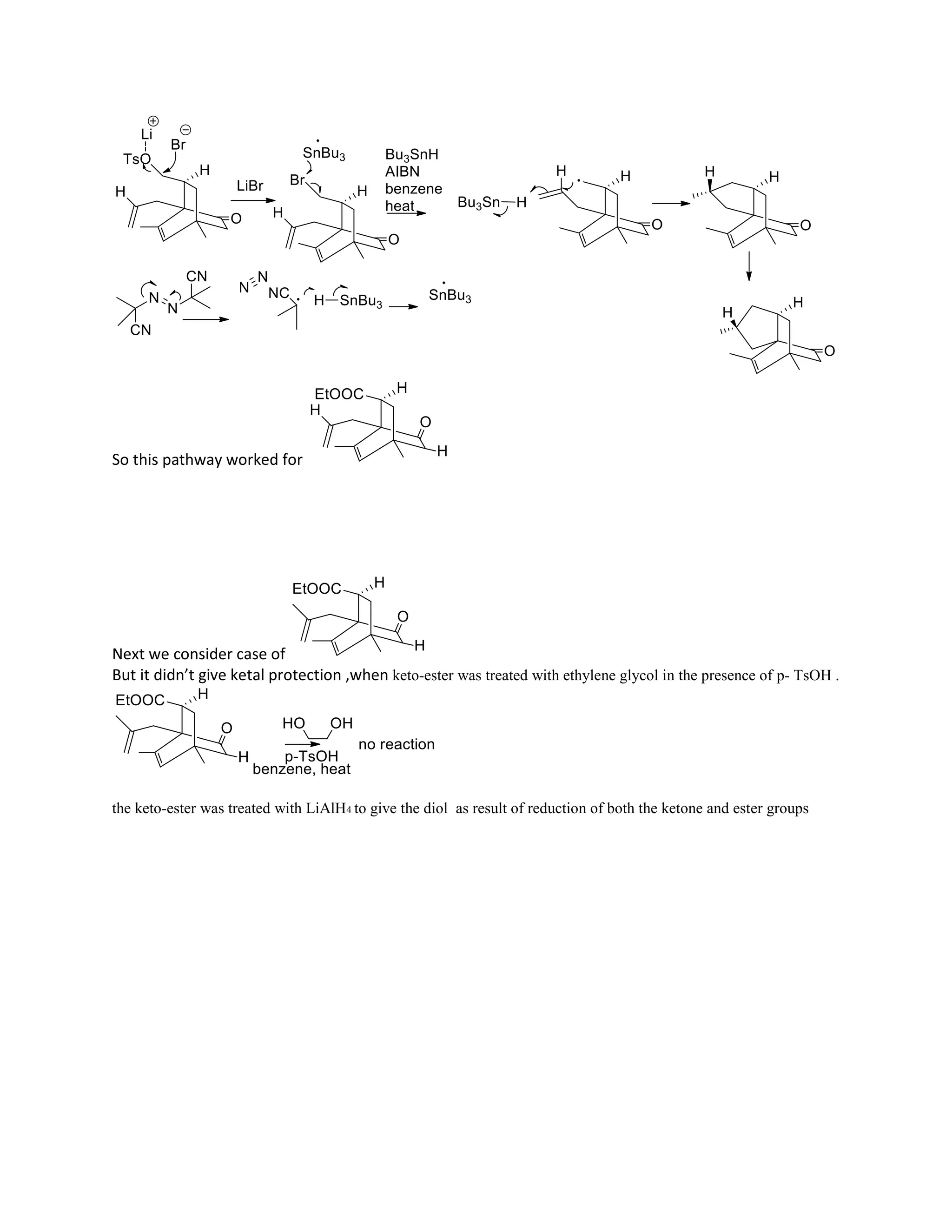
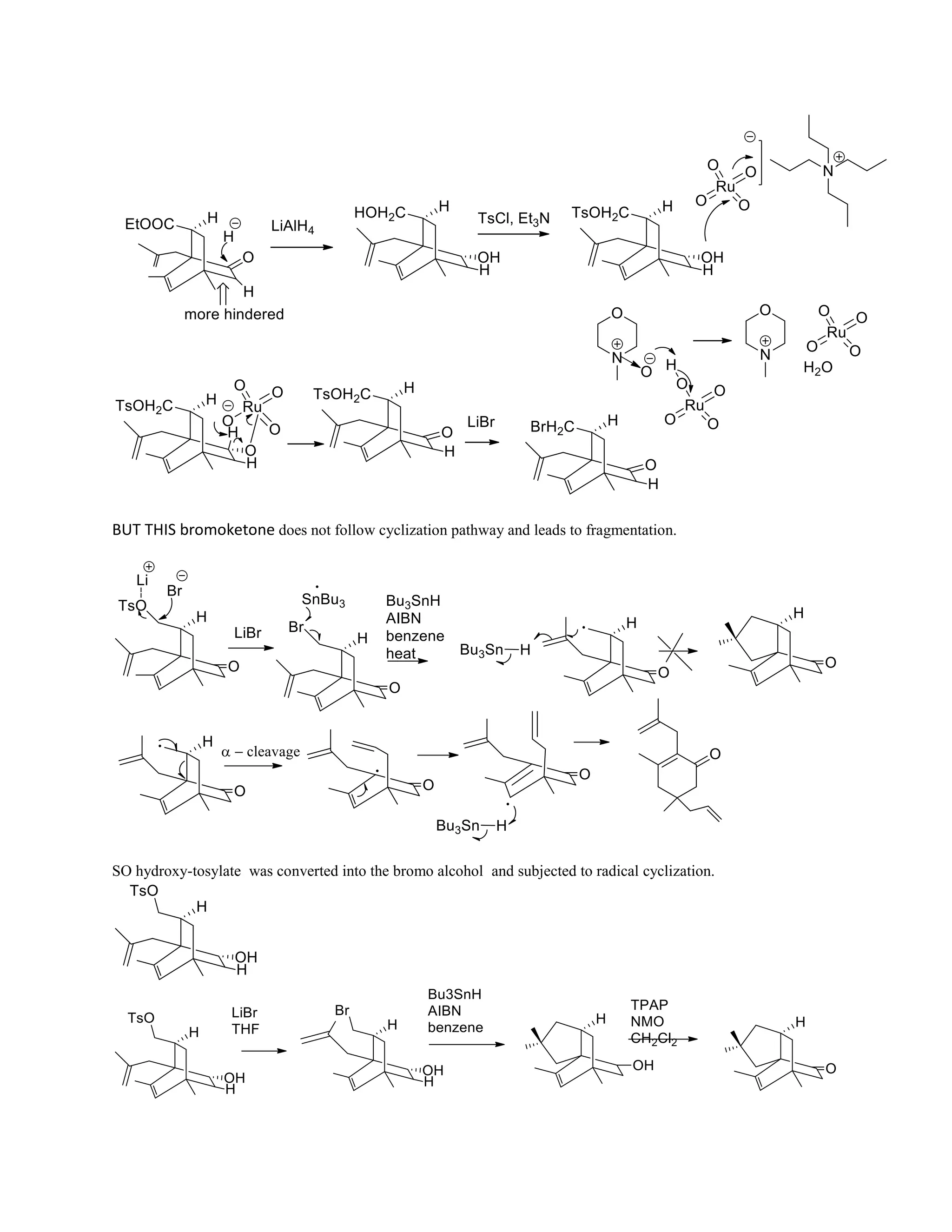
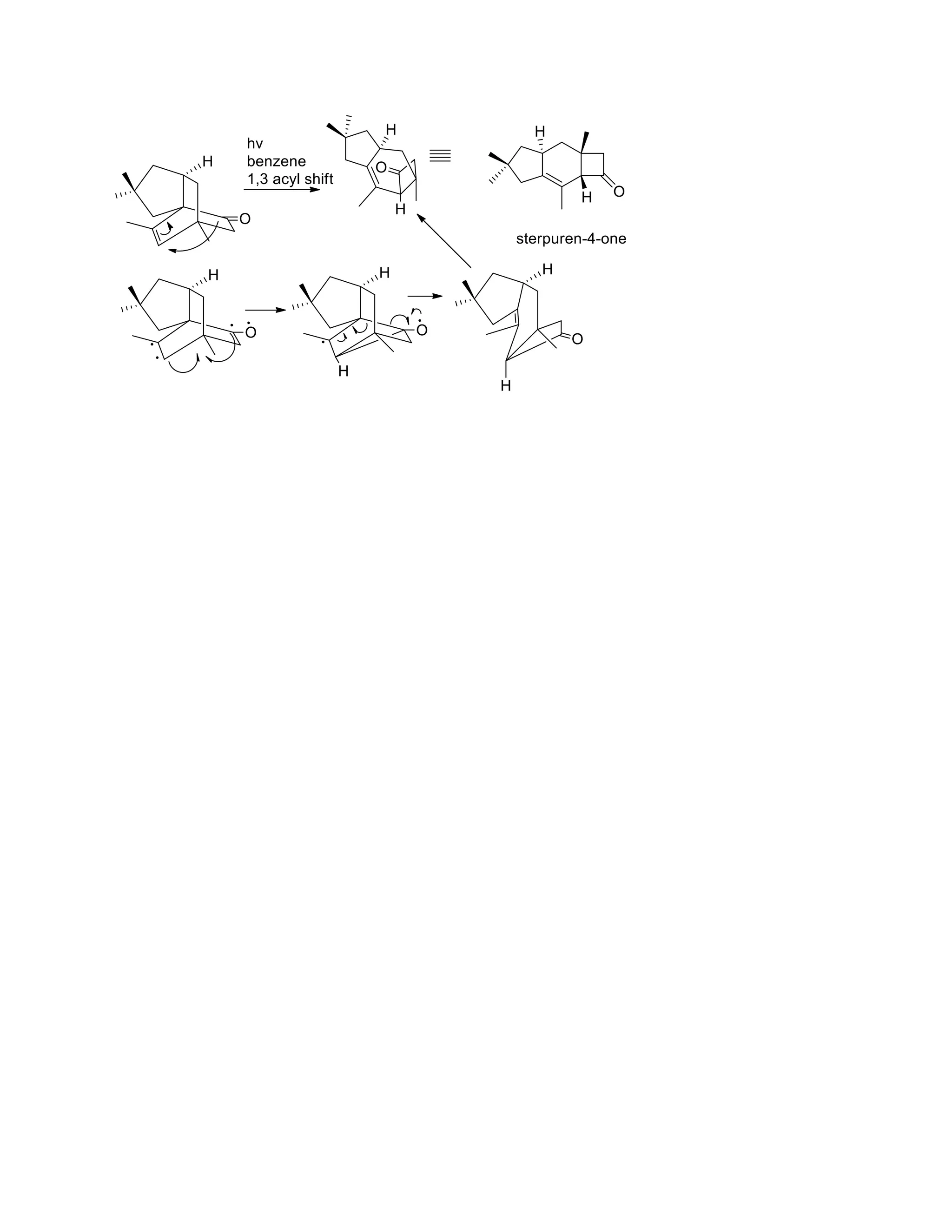
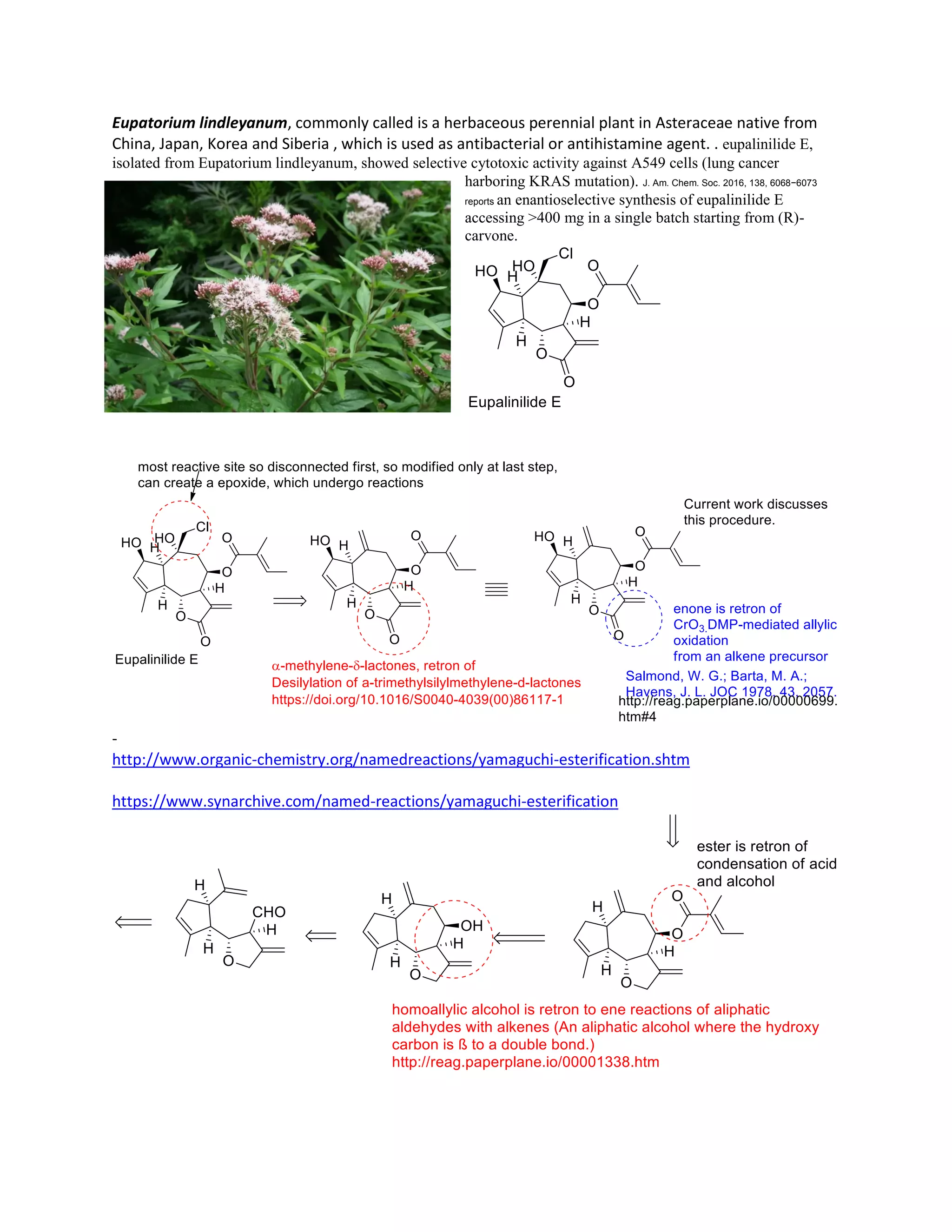
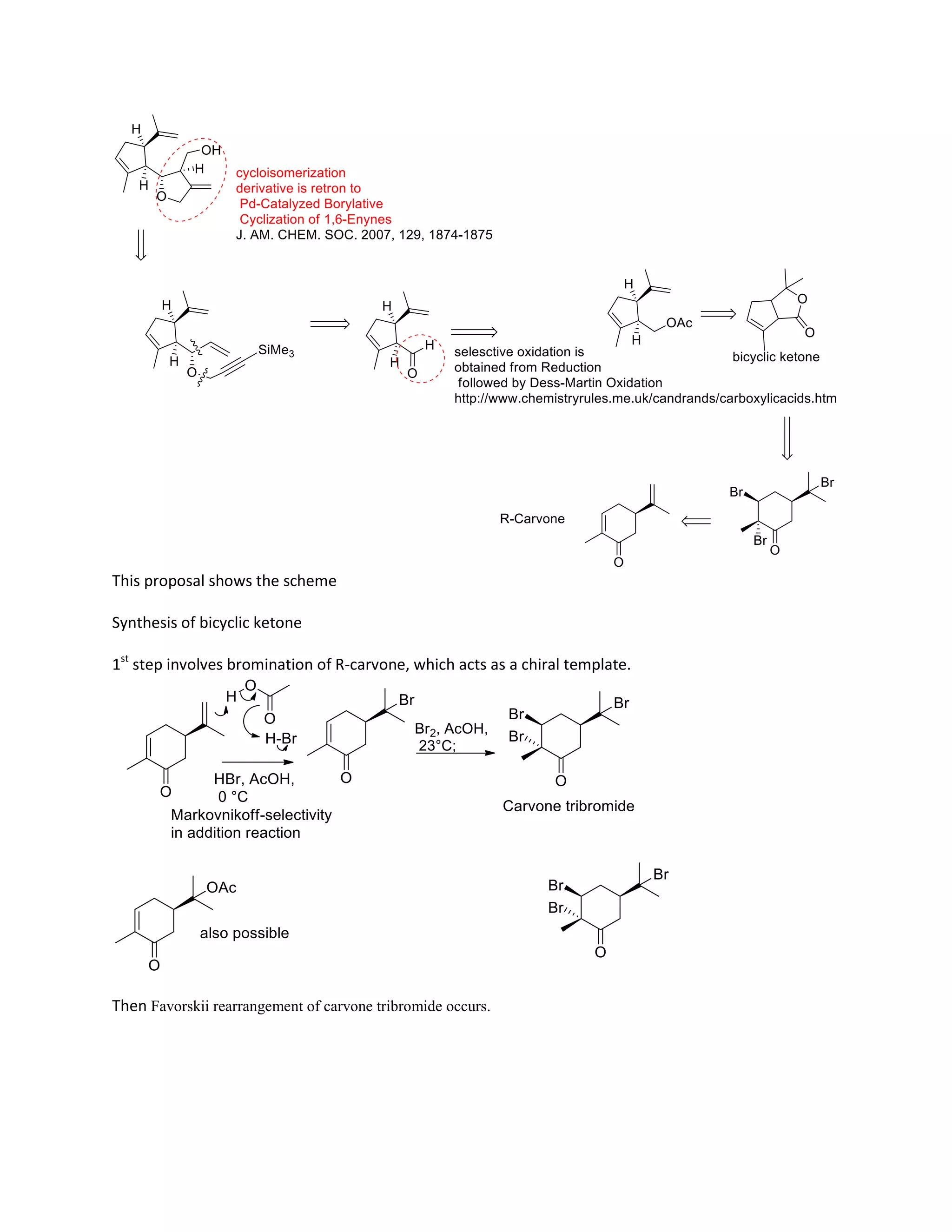
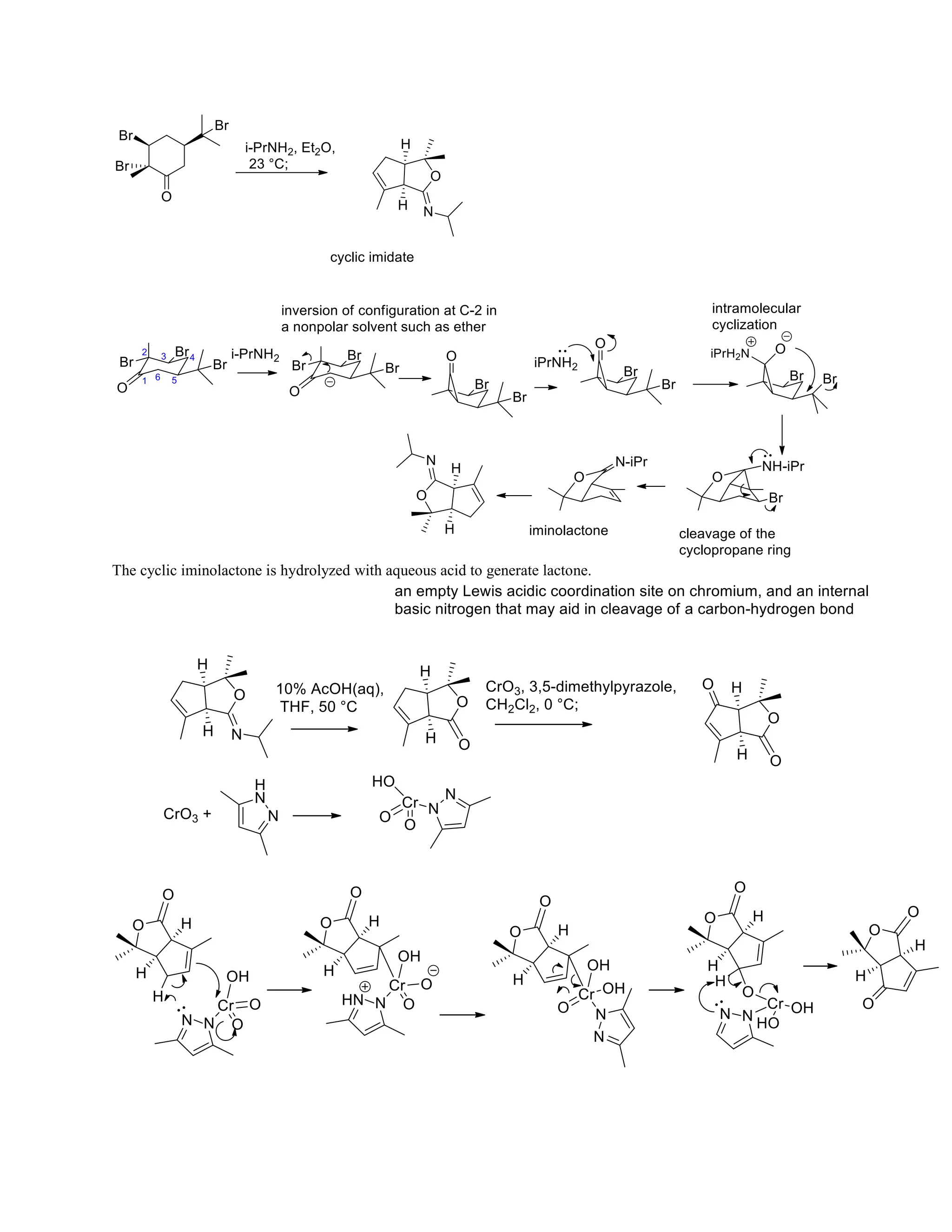
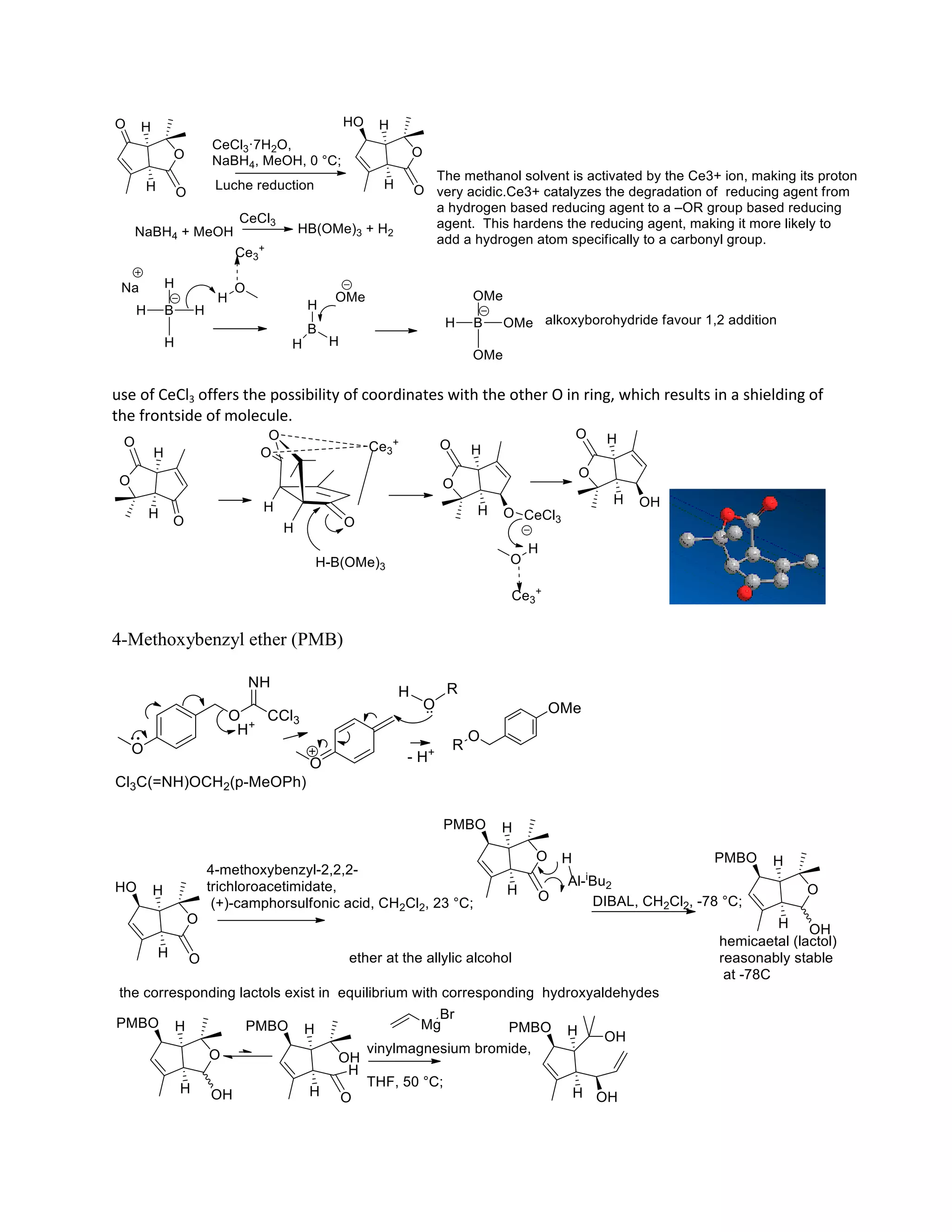
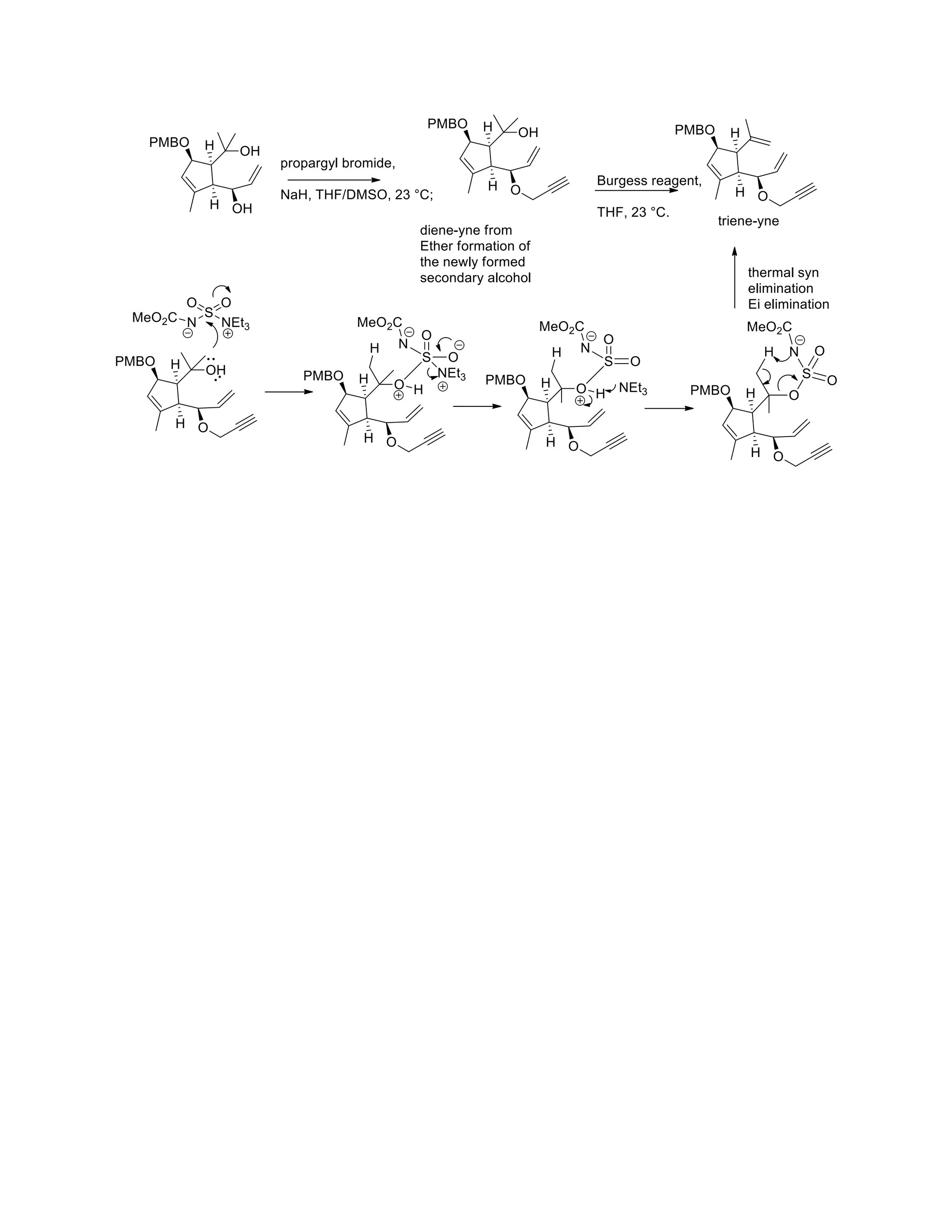
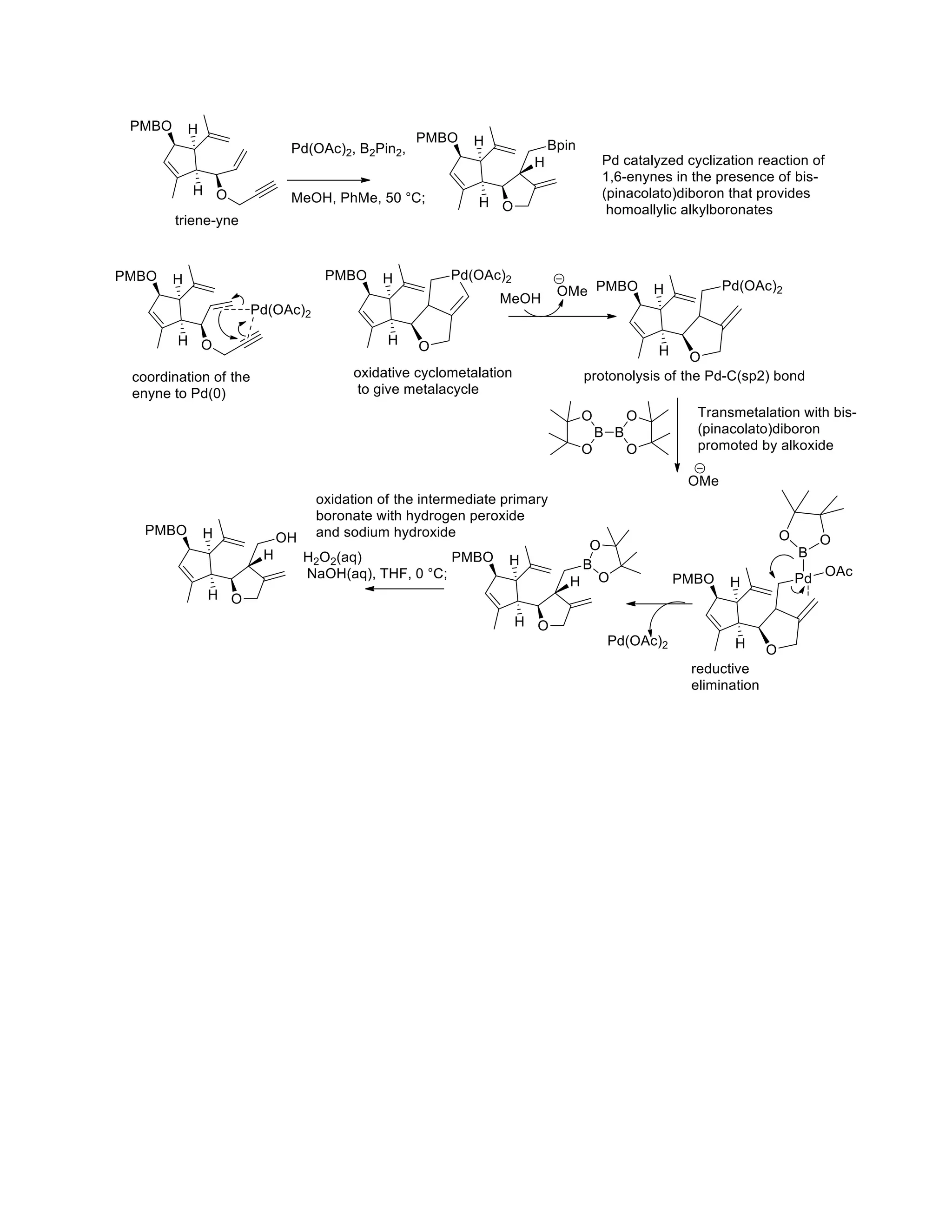
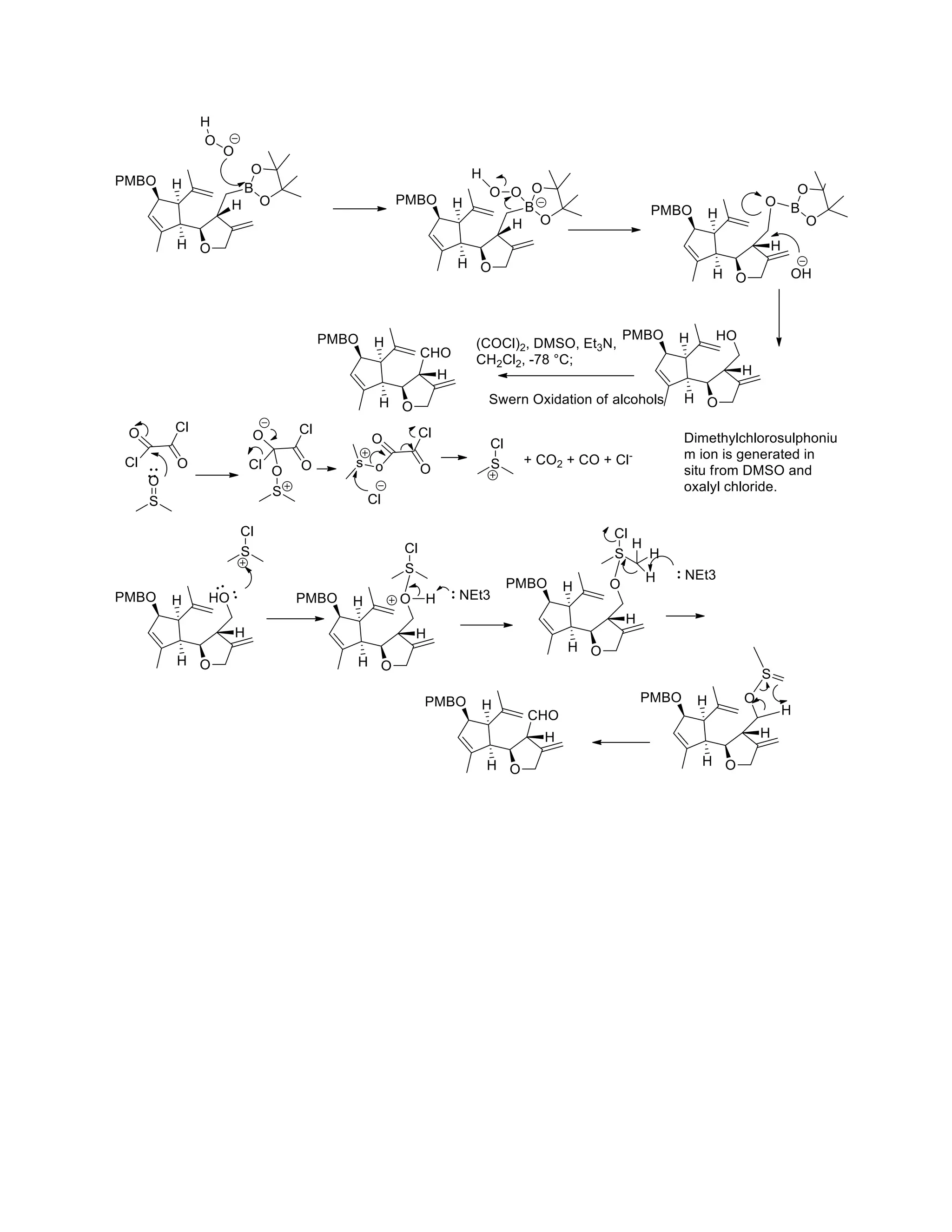
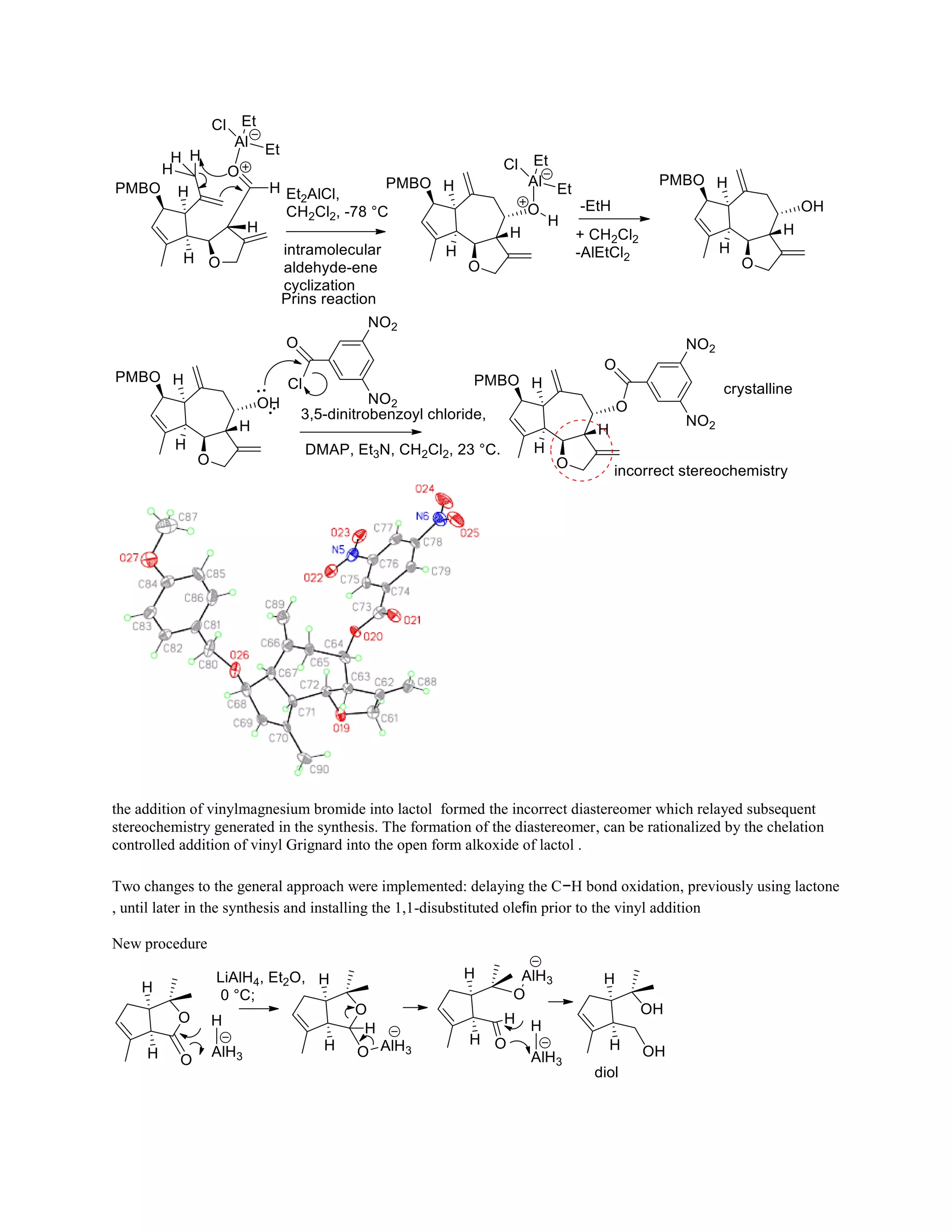
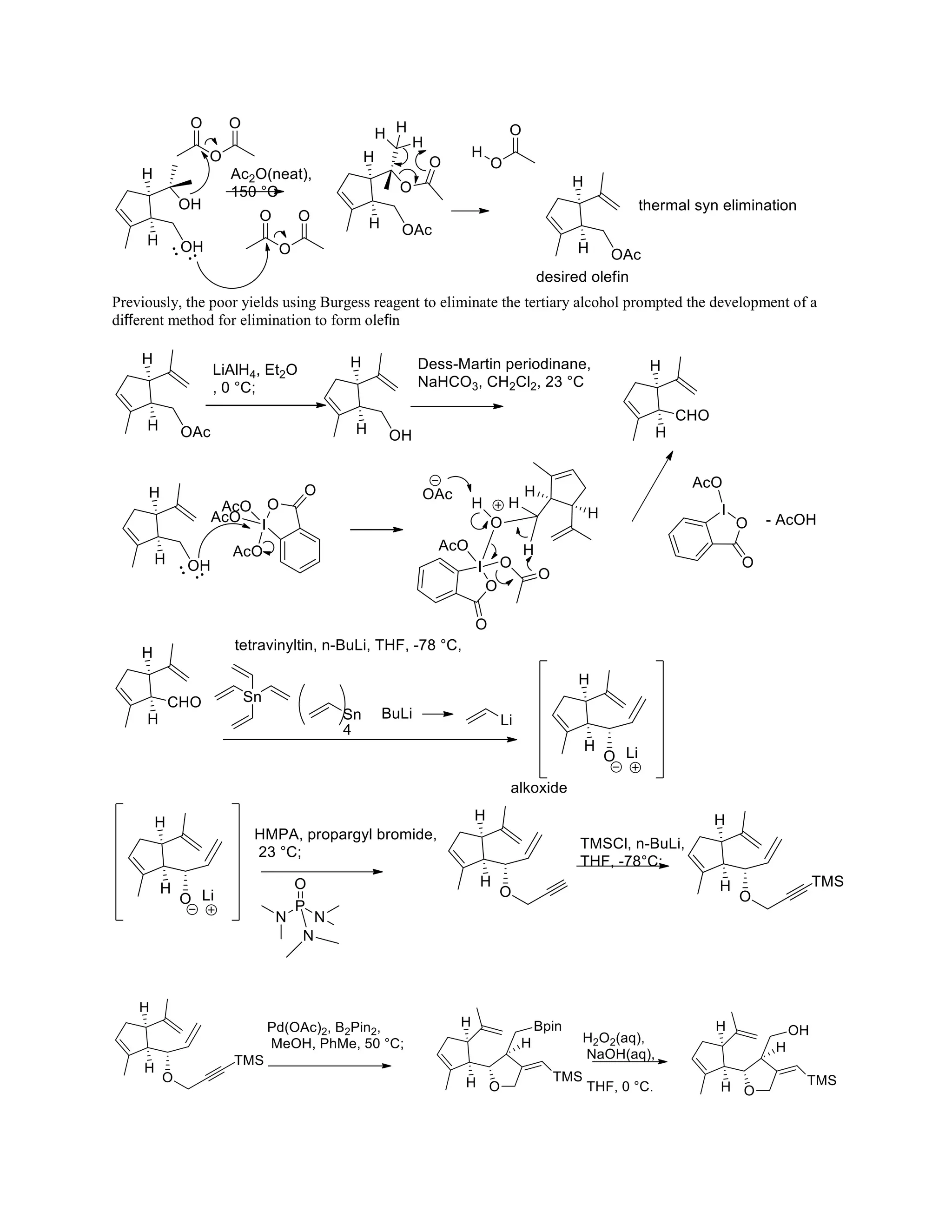
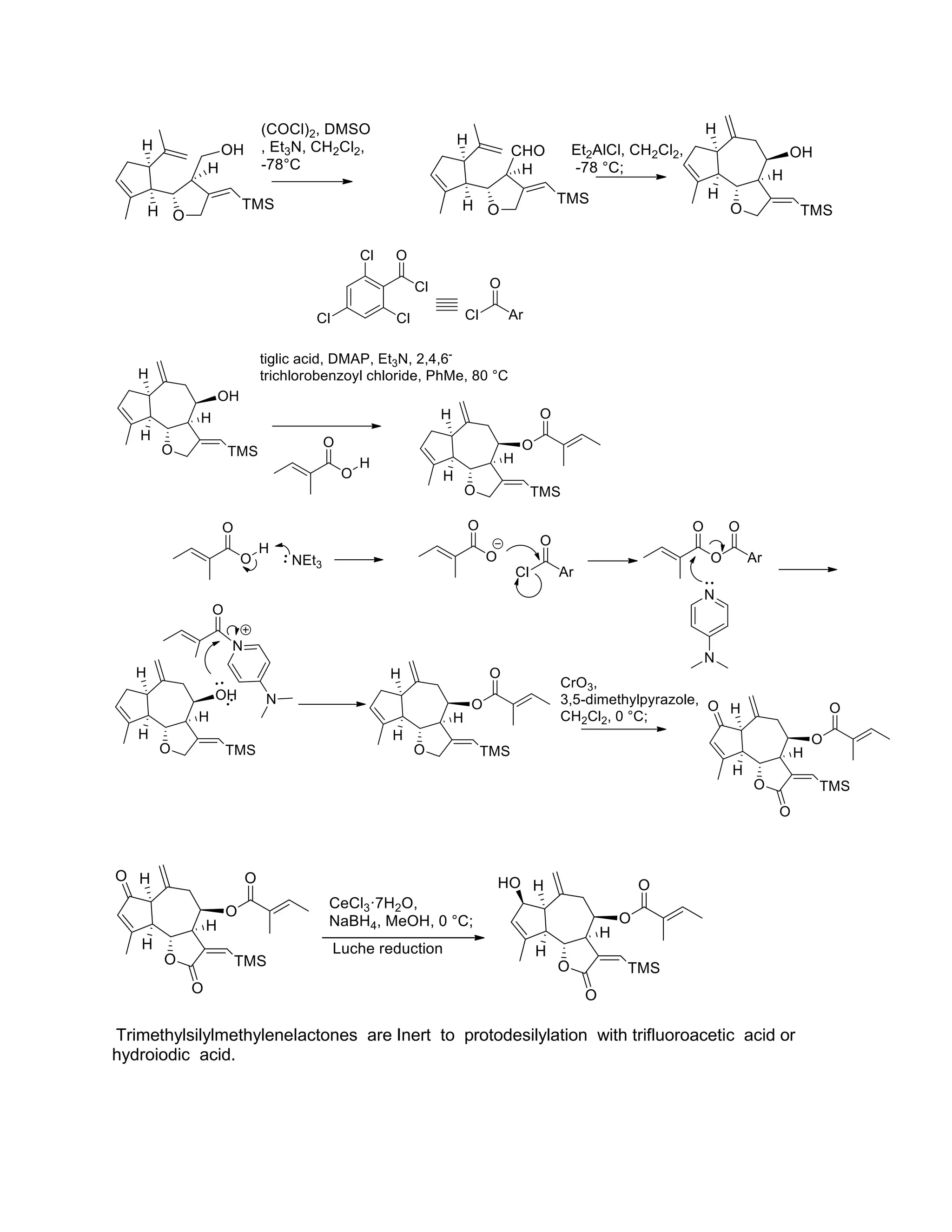
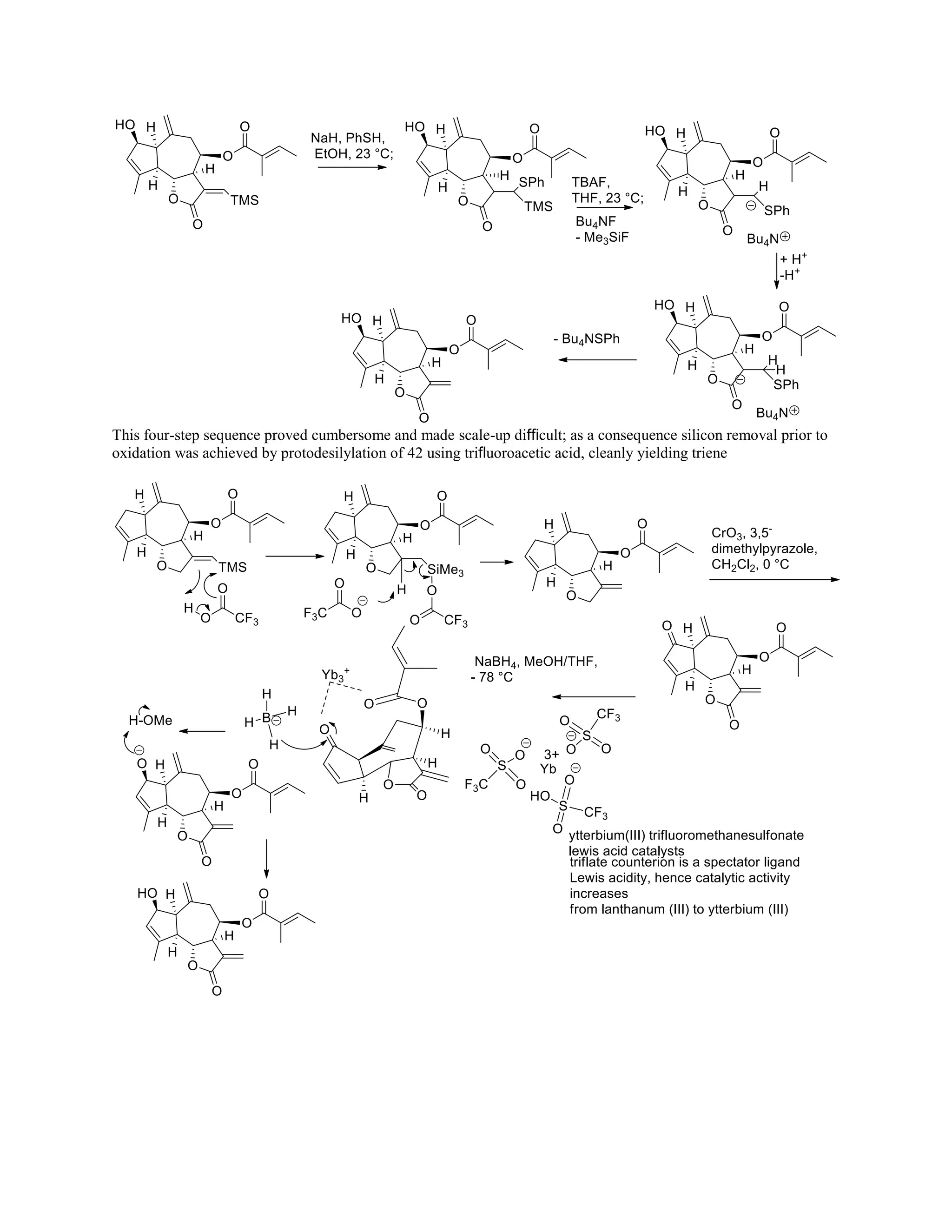
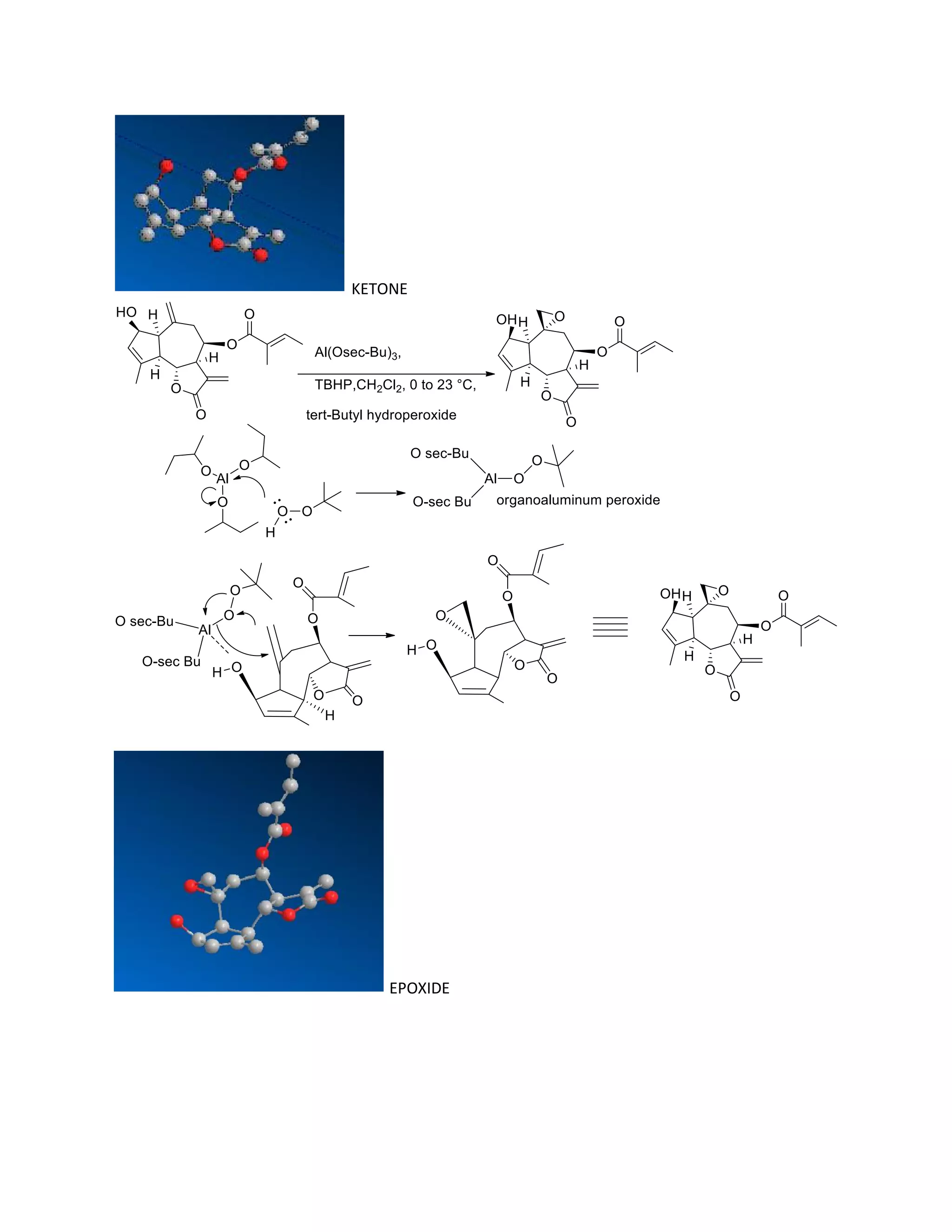
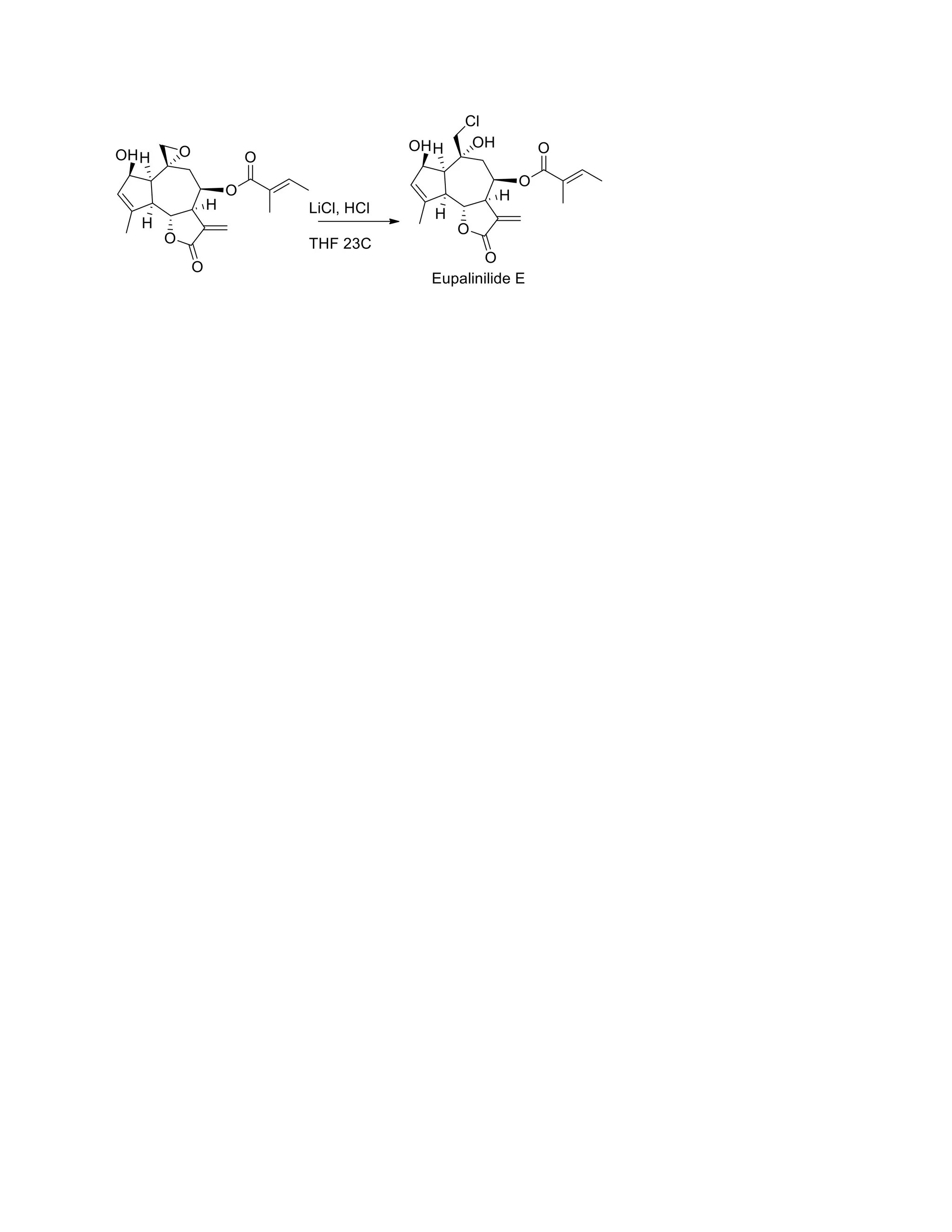
The document discusses various organic reaction mechanisms and total syntheses of specific molecules, highlighting methods such as enantioselective synthesis and catalytic reactions. It details the synthesis of compounds like (−)-pavidolide b and eupalinilide e, using strategies that involve chiral templates and specific reagents. Additionally, it notes challenges in synthesis procedures and adaptations made to improve reaction yields and efficiency.