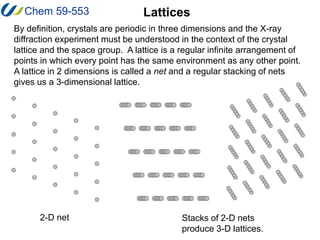
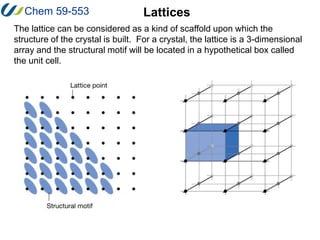
1. A lattice is a regular, infinite arrangement of points in which every point has the same environment as any other point. A 2D lattice is called a net and stacking of nets produces a 3D lattice.
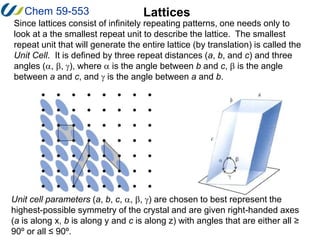
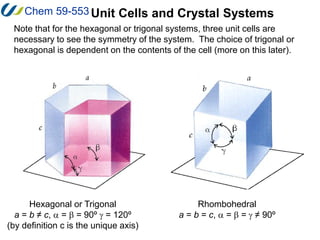
2. The unit cell is the smallest repeating unit that generates the entire lattice through translations. It is defined by three distances (a, b, c) and three angles (α, β, γ).
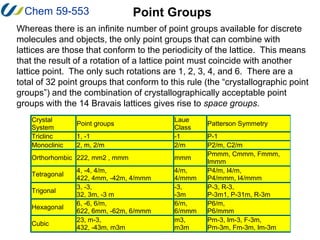
3. There are 14 possible lattice arrangements, or Bravais lattices, that result from the 7 crystal systems which place different restrictions on the unit cell parameters. Lattices can have different types of centering.