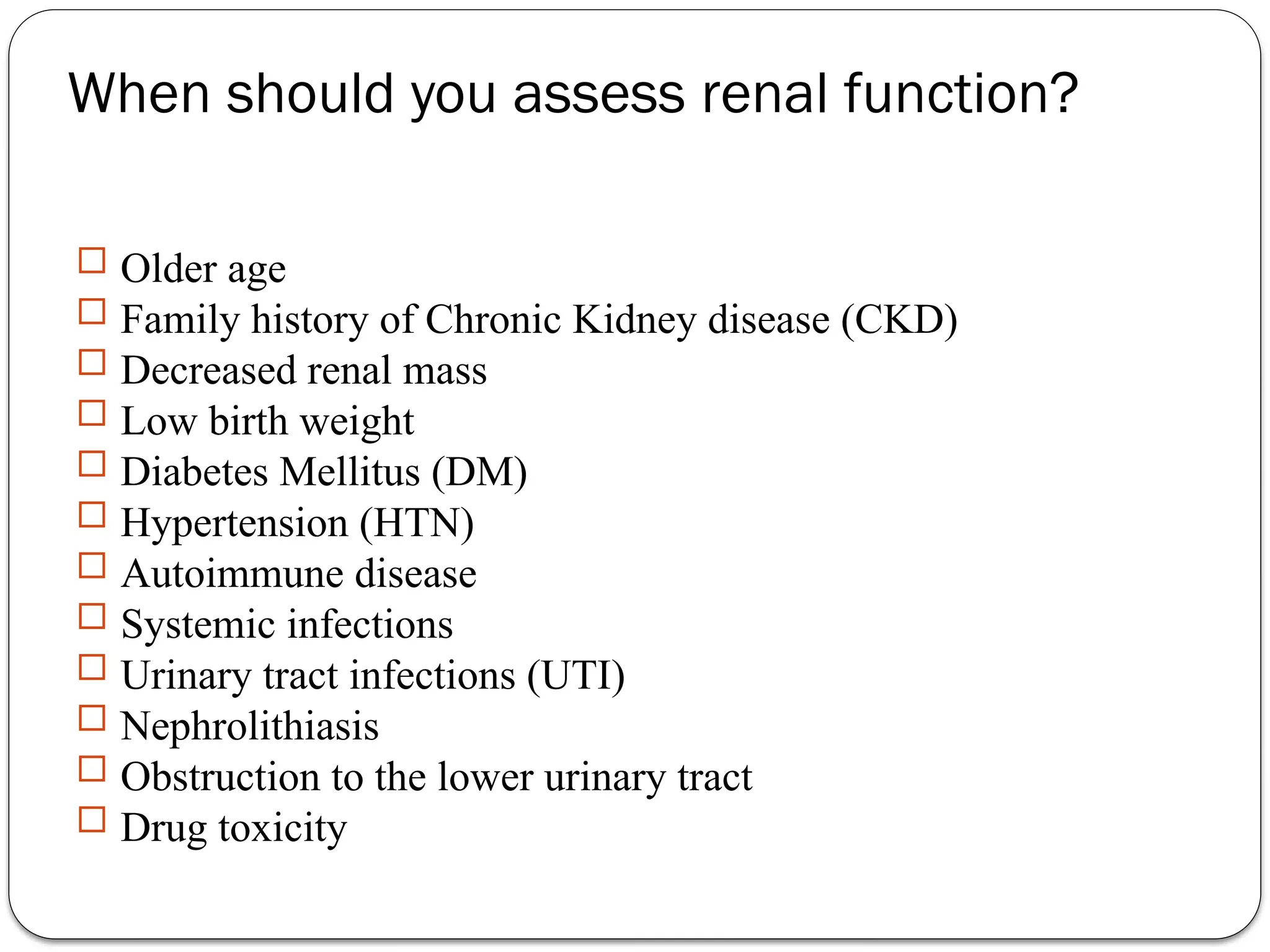
The document outlines various kidney function tests, including assessments of glomerular filtration rate (GFR) and renal tubular function. It emphasizes the importance of understanding when to evaluate renal function based on patient history and health conditions, primarily utilizing biochemical tests like creatinine clearance, plasma urea, and urinalysis. It also discusses the implications of abnormal results and conditions such as nephrotic syndrome and proteinuria.