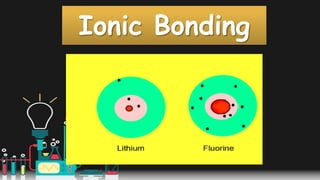
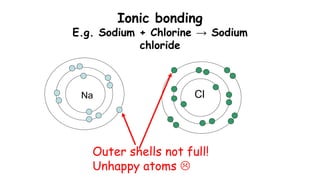
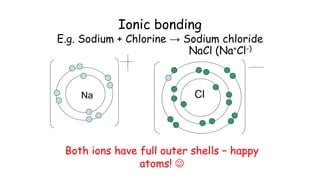
This document provides information about ionic bonding between atoms. It describes how ions are formed when atoms transfer electrons to obtain full outer electron shells. Sodium loses an electron to become sodium ion (Na+), and chlorine gains that electron to become chloride ion (Cl-), forming an ionic bond in sodium chloride (NaCl). Other examples of ionic bonding covered are beryllium fluoride (BeF2) and practice drawing Lewis structures for LiCl, K3N, BaS, and CaF2. The overall purpose is to explain how ionic bonds are formed through the transfer of electrons between atoms to achieve stable full outer shells.