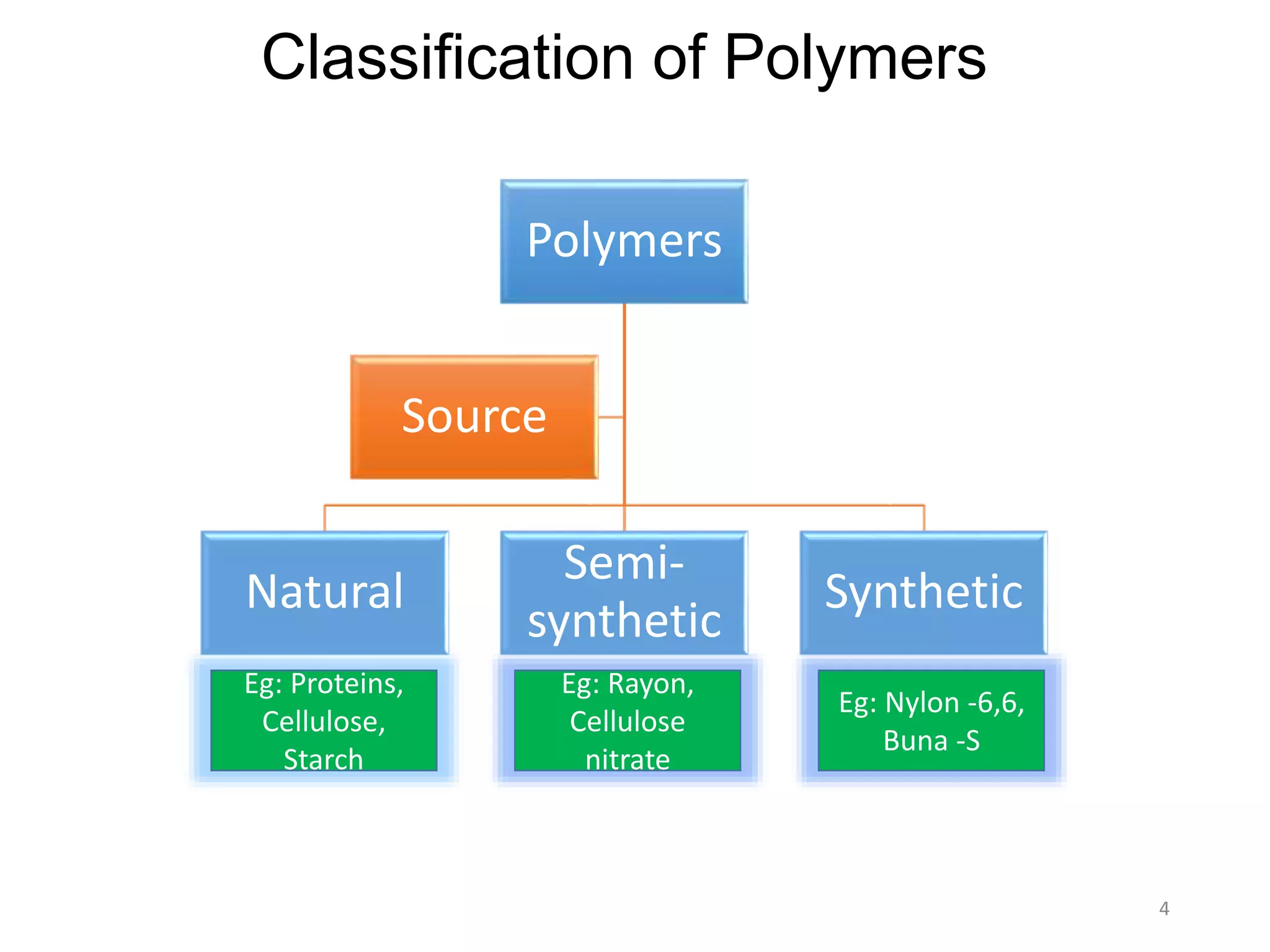
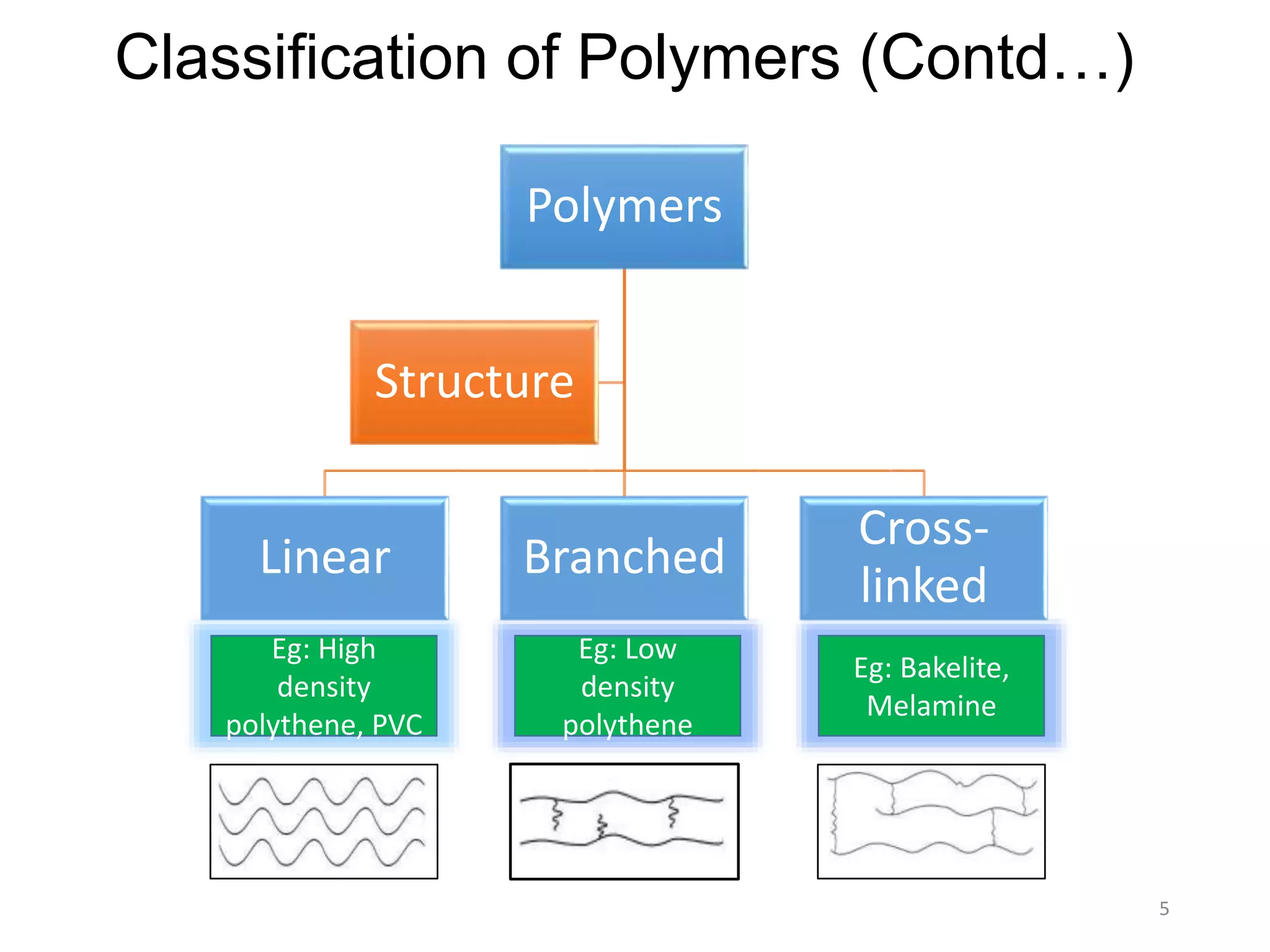
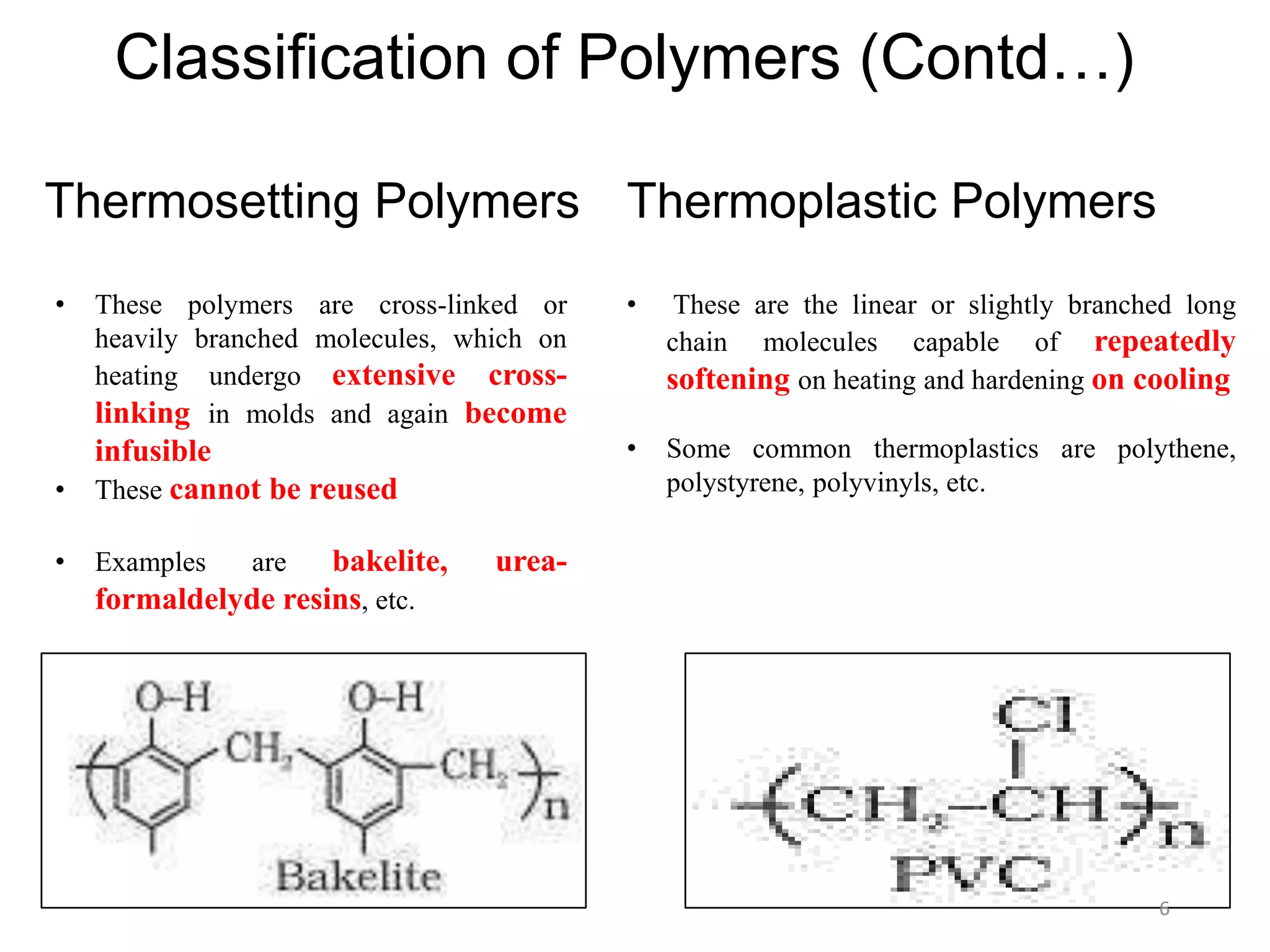
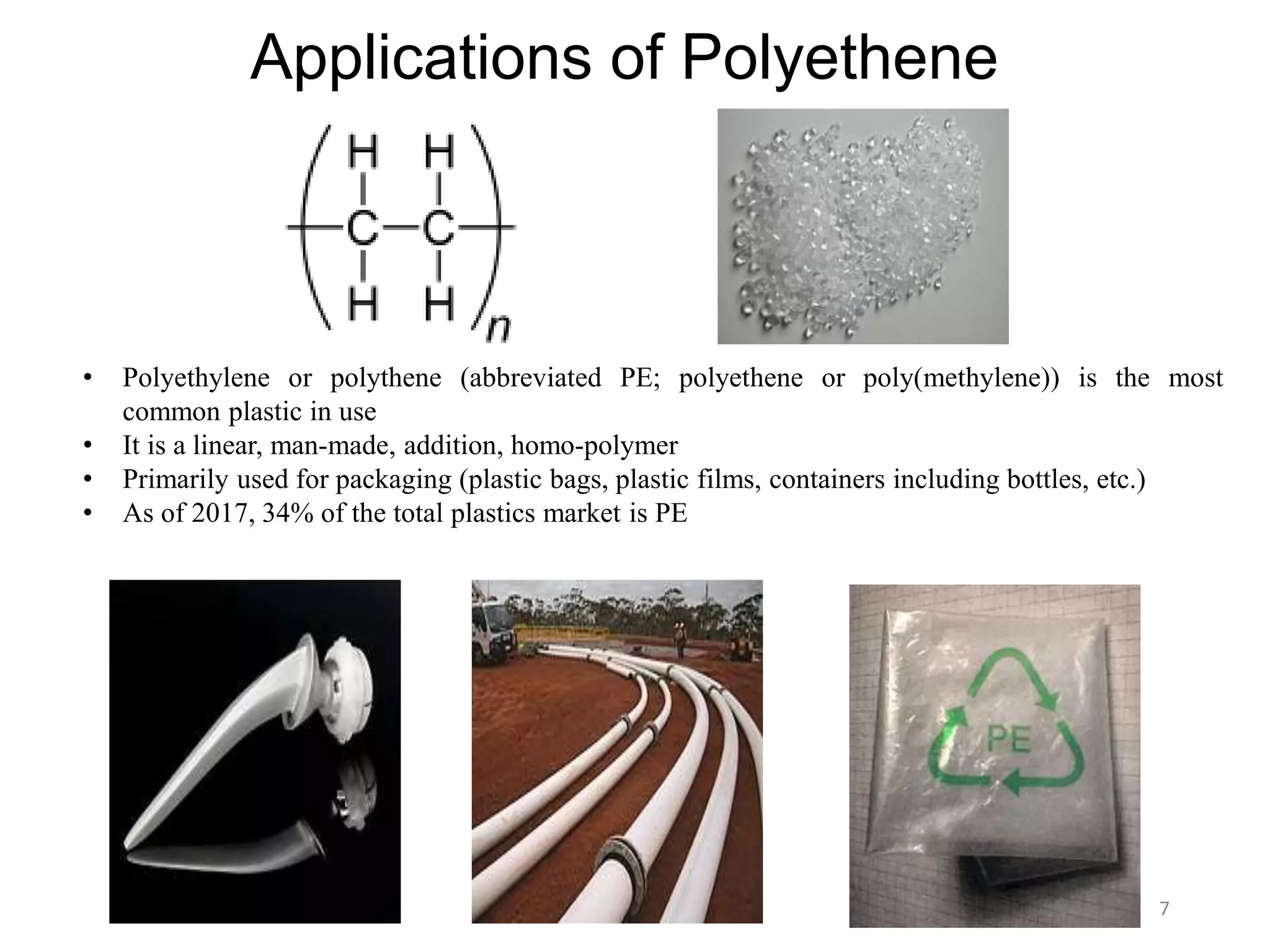
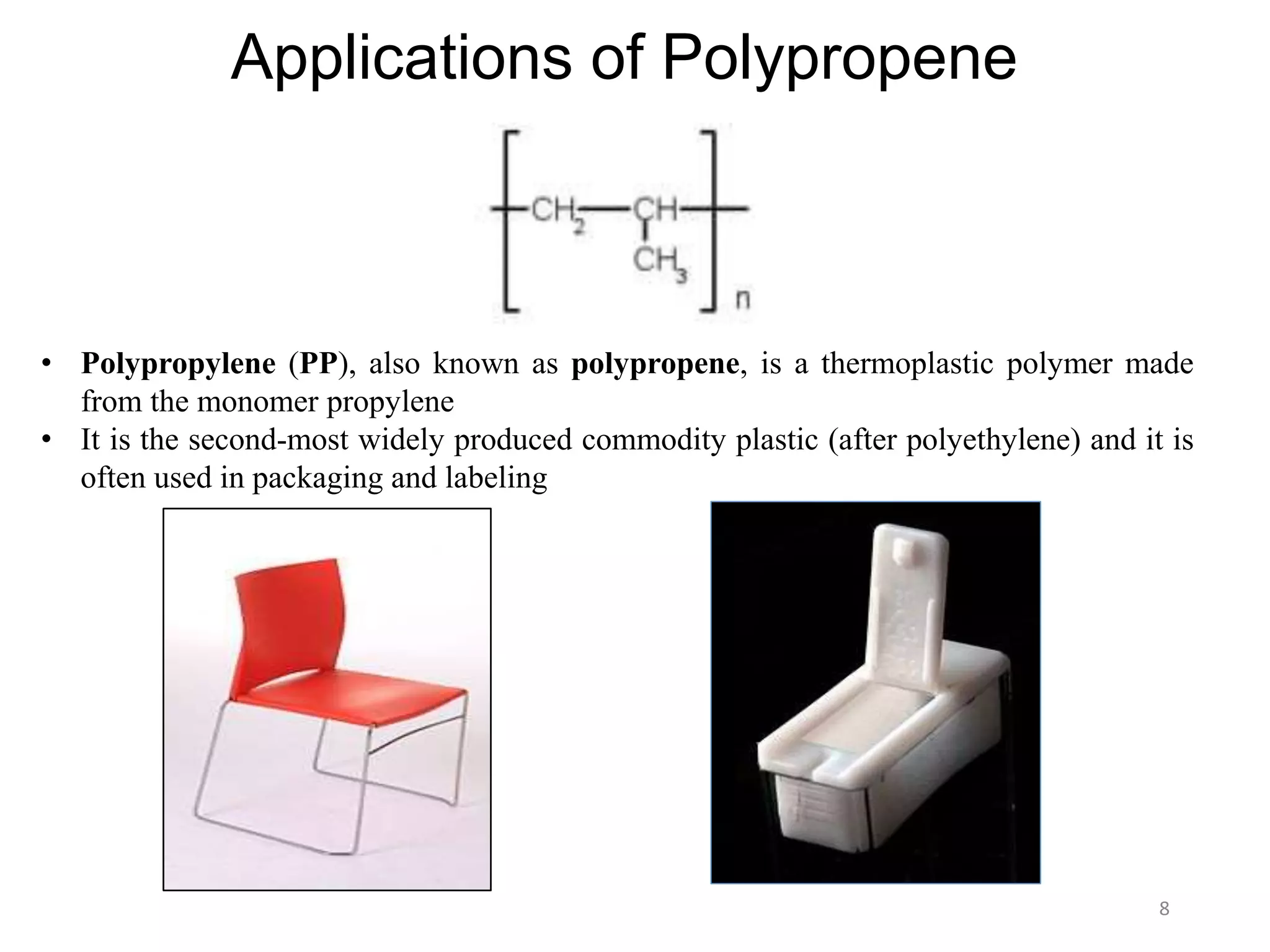
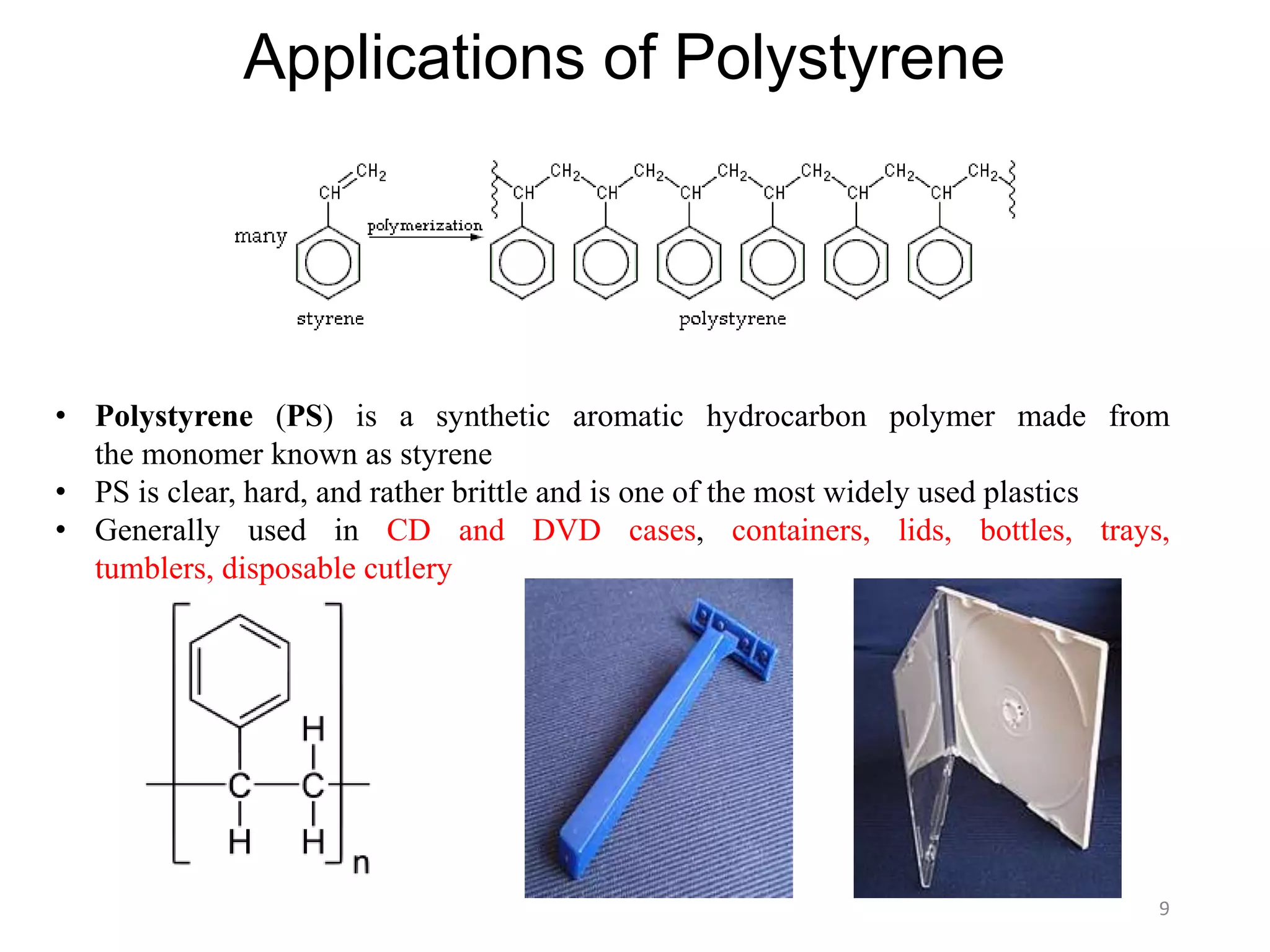
This document discusses polymers and their classification and applications. It begins with defining polymers as large molecules formed by linking repeating structural units through covalent bonds, in a process called polymerization. Polymers are classified as natural, semi-synthetic, or synthetic, and as linear, branched, or cross-linked. Thermoplastics are linear or slightly branched polymers that soften on heating and harden on cooling, while thermosettings are heavily cross-linked polymers that set during molding. Common applications described include polyethylene in packaging, polypropylene in packaging and labeling, and polystyrene in containers and disposable items.