Here are the answers to the cold call questions:
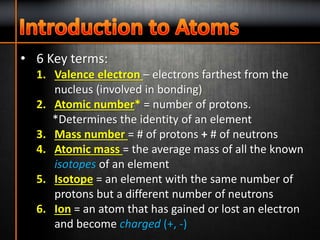
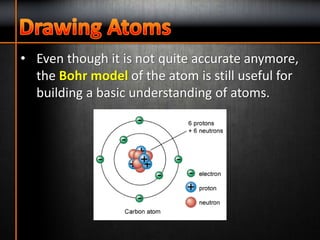
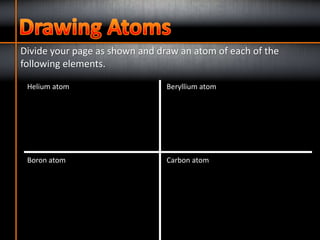
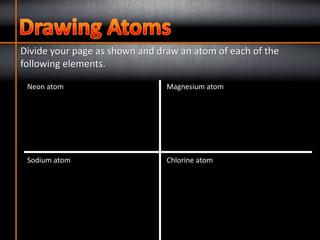
1. The three subatomic particles in an atom are protons (with a positive charge of +1), neutrons (with no charge), and electrons (with a negative charge of -1).
2. The two subatomic particles found in the nucleus of an atom are protons and neutrons.
3. Electrons are found orbiting the nucleus of an atom.
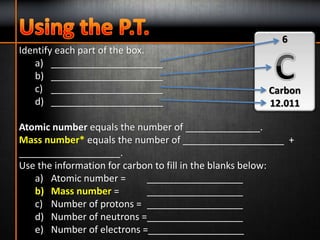
4. The number of protons determines the chemical element of an atom.
5. A valence electron is an electron in the outermost electron shell of an atom that is involved in bonding with other atoms.
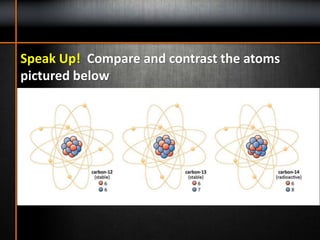
6. Atomic mass is the average mass of all the known isotopes of