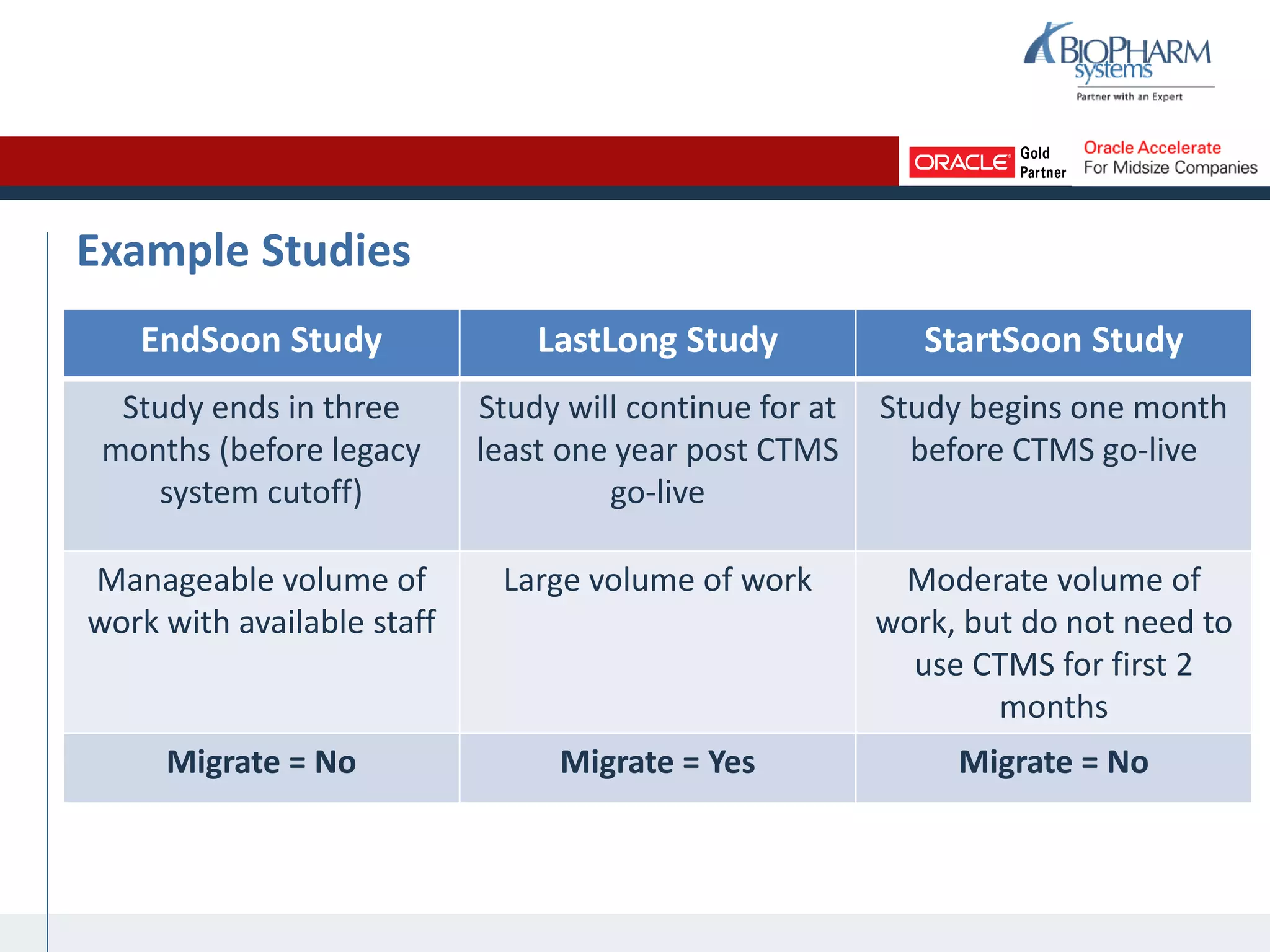
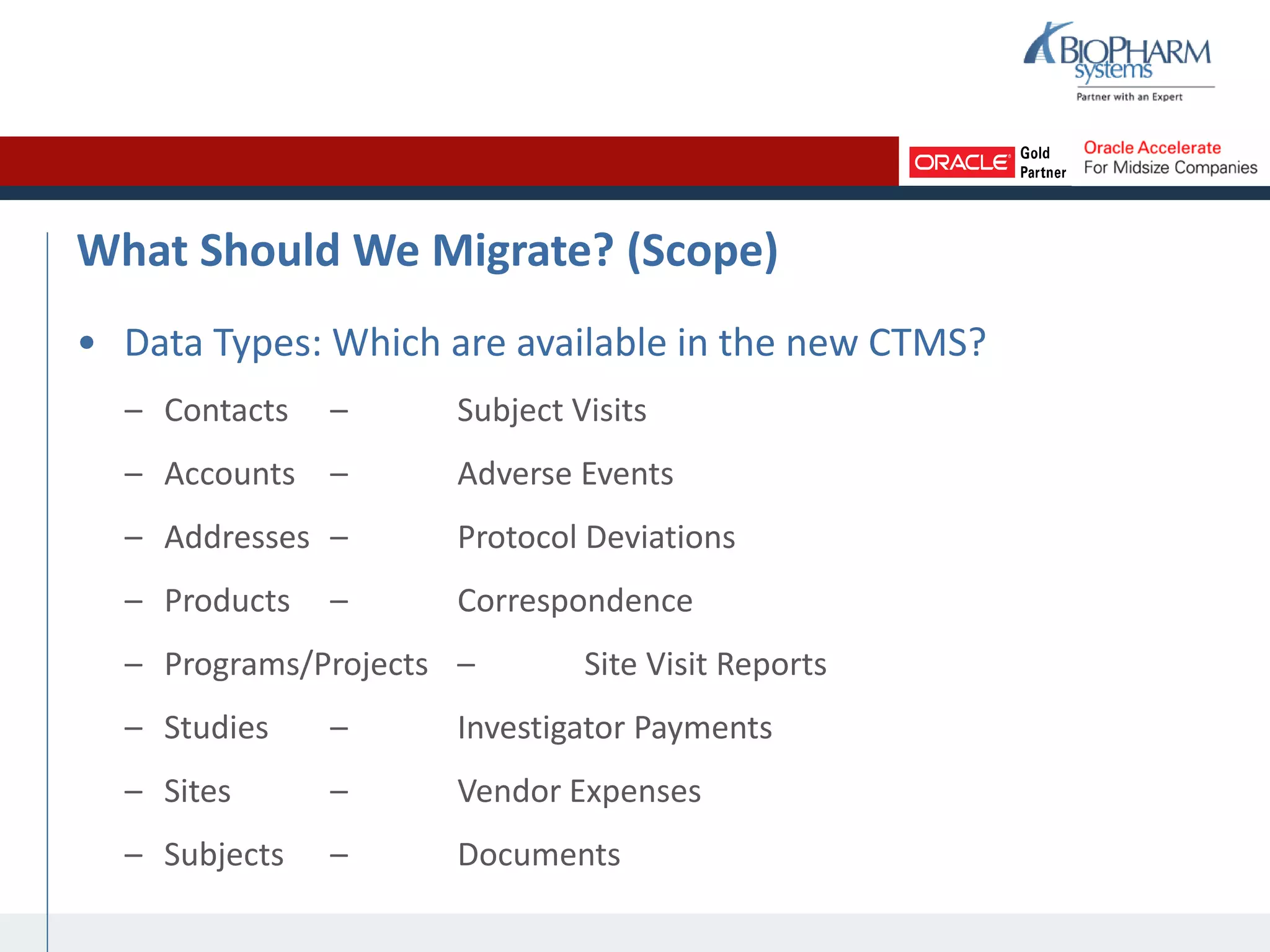
The document summarizes a presentation on CTMS data migration. It discusses four phases of analyzing a potential CTMS data migration: purpose, scope, methods, and timing. For purpose, it examines the business reasons for migrating and risks. For scope, it addresses determining which studies and data types to include. For methods, it evaluates tools and complexity. For timing, it considers aligning with the CTMS rollout strategy. The presentation provides examples and recommends a study-by-study migration approach to pilot the process before full implementation. It demos the ASCEND-Migrate tool and closes with Q&A.