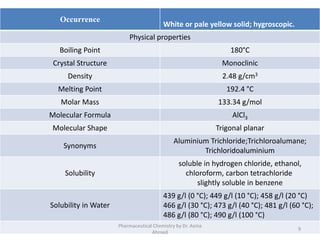
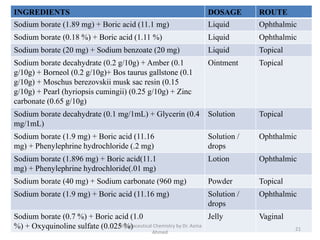
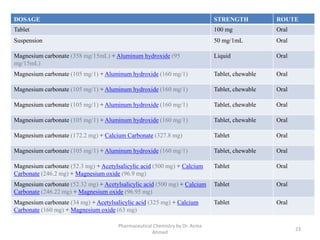
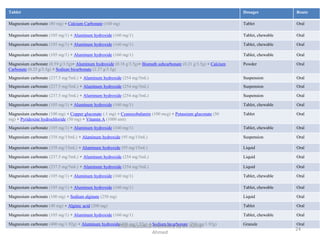
The document summarizes information about 5 inorganic drugs: aluminum hydroxide, aluminum chloride, sodium carbonate, sodium chloride, and sodium thiosulphate. It describes their occurrence, physical characteristics, pharmaceutical uses, incompatibilities, adverse effects, and preparations. Aluminum hydroxide is used as an antacid and adsorbent. Aluminum chloride is used as a hemostatic and antiperspirant. Sodium carbonate, sodium chloride and sodium thiosulphate are used for a variety of purposes including as emetics, douches, and cyanide poisoning antidotes. Their uses depend on their chemical properties and interactions.





































![– As a component of human milk calcium enters milk. Use caution
if breastfeeding.
• Adverse effect
– Redness
– Low blood magnesium (hypomagnesemia)
– Low blood phosphates (hypophosphatemia)
– Low blood pressure (hypotension)
– High blood calcium (hypercalcemia)
– Nausea
– Tissue necrosis at injection site
– Vasodilation
– Weakness
– Kidney stones
– Hot flashes
– Serum amylase increased
– Tingling sensations
– Injection site reactions (tingling, burning sensation, inflammation of the veins
[phlebitis])
Pharmaceutical Chemistry by Dr. Asma
Ahmed
46](https://image.slidesharecdn.com/inorganicdrugs-230525152449-607dc72c/85/INORGANIC-DRUGS-pptx-46-320.jpg)