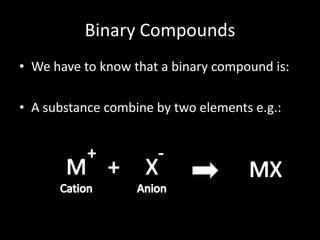
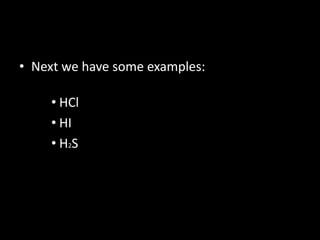This document introduces Marco Antonio Orozco de la Cruz, a chemical engineer and teacher from Guadalajara University who has been teaching chemistry, physics, and mathematics for teens and adults. It provides an overview of binary compounds, including how they are classified and named using both traditional and IUPAC nomenclature. Examples are given of naming acid compounds specifically, such as hydrogen chloride under traditional naming and hydrogen chloride under IUPAC naming. Readers are invited to contact Marco Antonio with any questions.